
A release scheduled for Monday, January 26, 2026, will implement changes to align the Prior Approval Change of PD/PI Request, Just-in-Time (JIT), and Research Performance Progress Report (RPPR) Participants section with the Common Forms requirements detailed in NOT-OD-26-018. The use of the Biographical Sketch and Current and Pending (Other) Support (CPOS) Common Forms and the NIH Biographical Sketch Supplement will be required for all individuals required to submit one of the above documents, including all Senior/Key personnel, on or after January 25, 2026.
These changes in Prior Approval, JIT, and RPPR will require all Senior/Key and any new Other Significant Contributor participants on an application or award to have an active CommonsID which is linked to their ORCID iD. The ORCID iD will be used as the unique identifier in SciENcv to produce certified PDF forms for participants’ Biosketches and CPOS forms. For information on how to link your ORCID iD to your CommonsID please visit the video Link Your ORCID iD to Your eRA Commons Account.
Overall Changes for Participants:
- All people listed as Senior/Key Personnel or New Other Significant Contributors must have a CommonsID and that CommonsID must be linked to the ORCID iD they use in SciENcv
- All Biographical Sketch and CPOS Common Form documents must be created in SciENcv and certified by the owner of the information
- When required, all Biographical Sketch and CPOS Common Form documents must be uploaded in the PDF format generated by SciENcv.
- The file name of the PDF may be updated once certified and downloaded from SciENcv. No other alterations may be made to the PDF. Do not flatten the PDF once certified and downloaded from SciENcv
- Users who have initiated but not submitted their Change of PD/PI Prior Approval or JIT as of January 25,2026 will need to redo the submission.
- Users who have initiated but not submitted their RPPR as of January 25, 2026, will need to redo the Participants section.
NOTE: Due to eRA’s deployment of updated user interfaces for implementation of the Common Forms for Biographical Sketch, Current and Pending (Other) Support (CPOS) and NIH Biographical Sketch Supplement:
Changes for Prior Approval – Change of PD/PI Request
Users must now upload SciENcv-created and certified, not-flattened PDF documents for the Biographical Sketch/NIH Biographical Sketch Supplement and CPOS for any new PD/PI being requested. If the PD/PI has Supporting Documentation that needs to be reported with their CPOS (e.g., copies of contracts specific to foreign appointments and/or employment with a foreign institution), it must be uploaded separately as a flattened PDF file in the Foreign Contract Document attachment field. The Common Form files will be validated to ensure accuracy and compliance; see Figure 1.
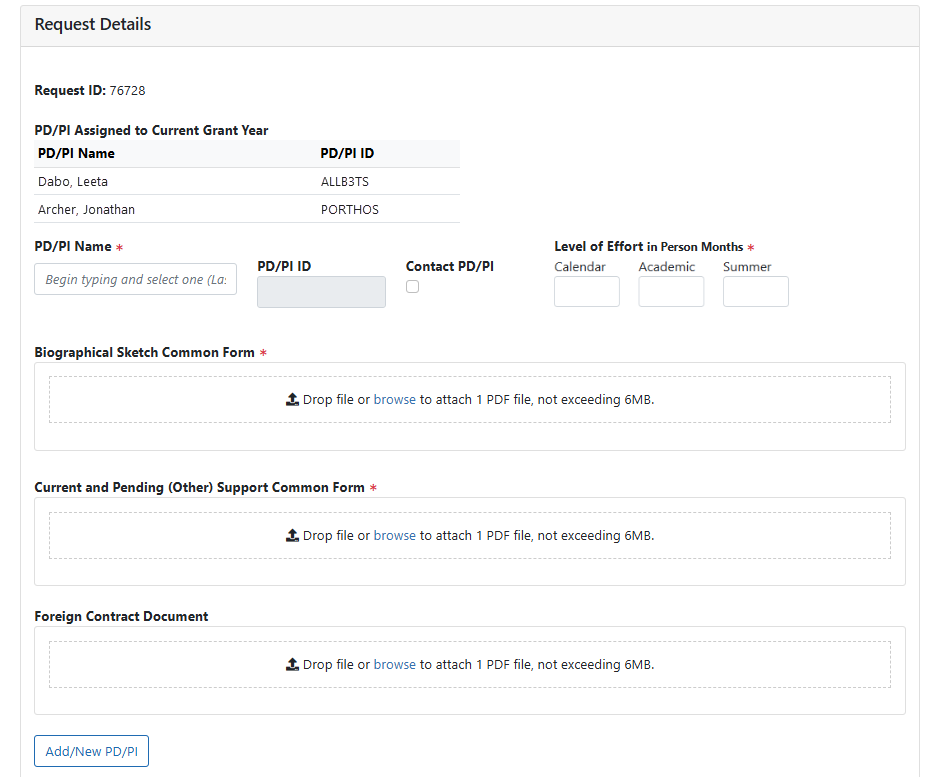
Figure 1: Prior Approval – Change of PD/PI Request details with Common Forms required.
Changes for JIT
All Current and Pending (Other) Support documents or Biographical Sketch/NIH Biographical Sketch Supplement updates must now be SciENcv-created and certified PDF documents. If a Foreign Contract Supporting Document is needed it must be uploaded separately as a flattened PDF file. A new Biographical Sketch Common Form, Current and Pending (Other) Support Common Form, and Foreign Contract CPS section will be available for all JITs. The Common Form files will be validated to ensure accuracy and compliance; see Figure 2.
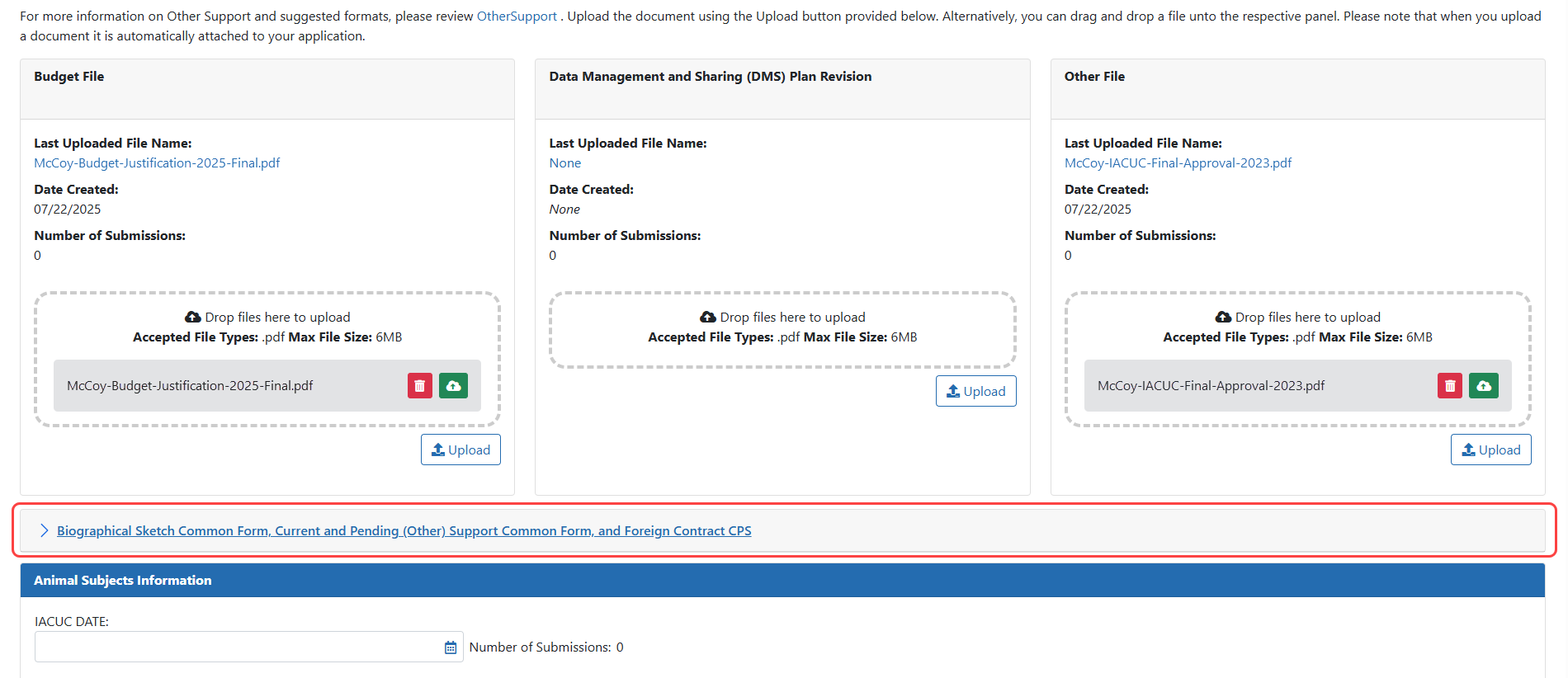
Figure 2: New Biographical Sketch Common Form, Current and Pending (Other) Support Common Form, and Foreign Contract CPS section in JIT.
Expansion of the new Biographical Sketch Common Form, Current and Pending (Other) Support Common Form, and Foreign Contract CPS section will allow users to Add Senior / Key Personnel details for each Senior/Key person. An eRA Commons User ID, ORCID and Name are required for each person.
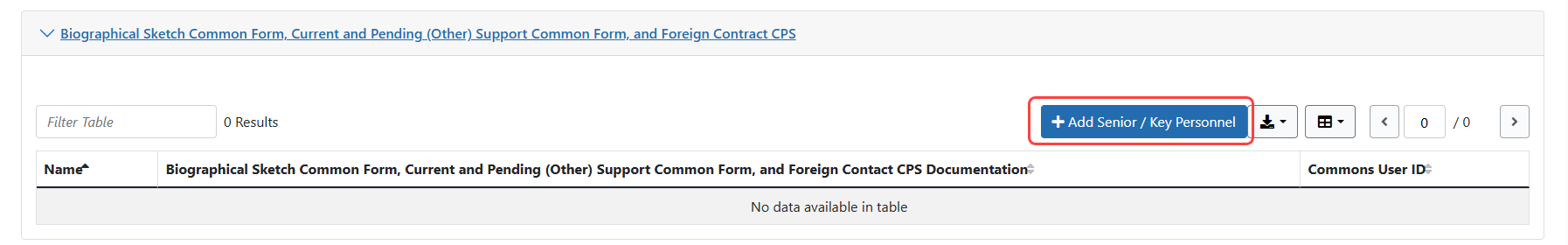
Figure 3: Expanded Biographical Sketch Common Form, Current and Pending (Other) Support Common Form, and Foreign Contract CPS section in JIT where the Add Senior/Key Personnel button is located.
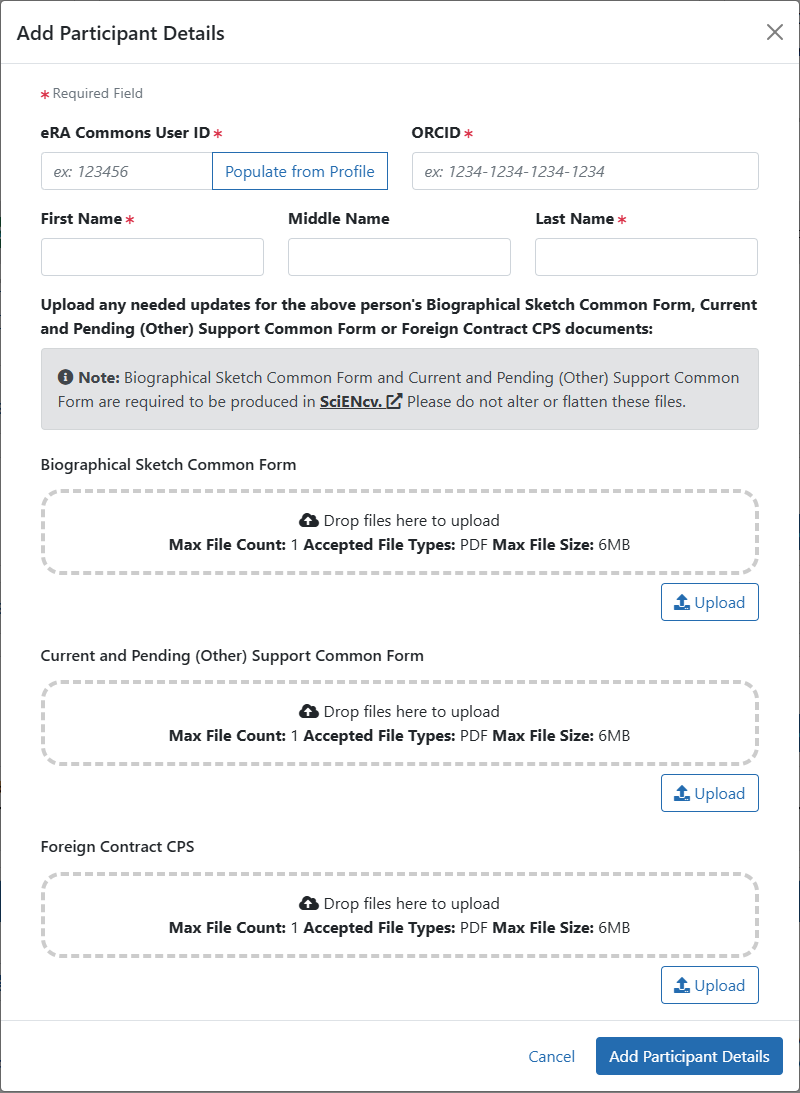
Figure 4: Add Participant Details modal where Senior/Key Personnel details are added including the eRA Commons User ID, ORCID, Name and Common Form PDFs.
Changes for RPPR – Section D. Participants
To accommodate the implementation of the Common Forms, the RPPR Participants section user interface will be updated to move questions related to New Senior/Key Personnel, Changes in Senior/Key Personnel Current and Pending (Other) Support, and New Other Significant Contributors (currently Questions D.2.b, D.2.c, and D.2.d respectfully) into the Participant Modal. The modal will responsively present questions and require file uploads based on the users’ answers; see Figures 5-8.
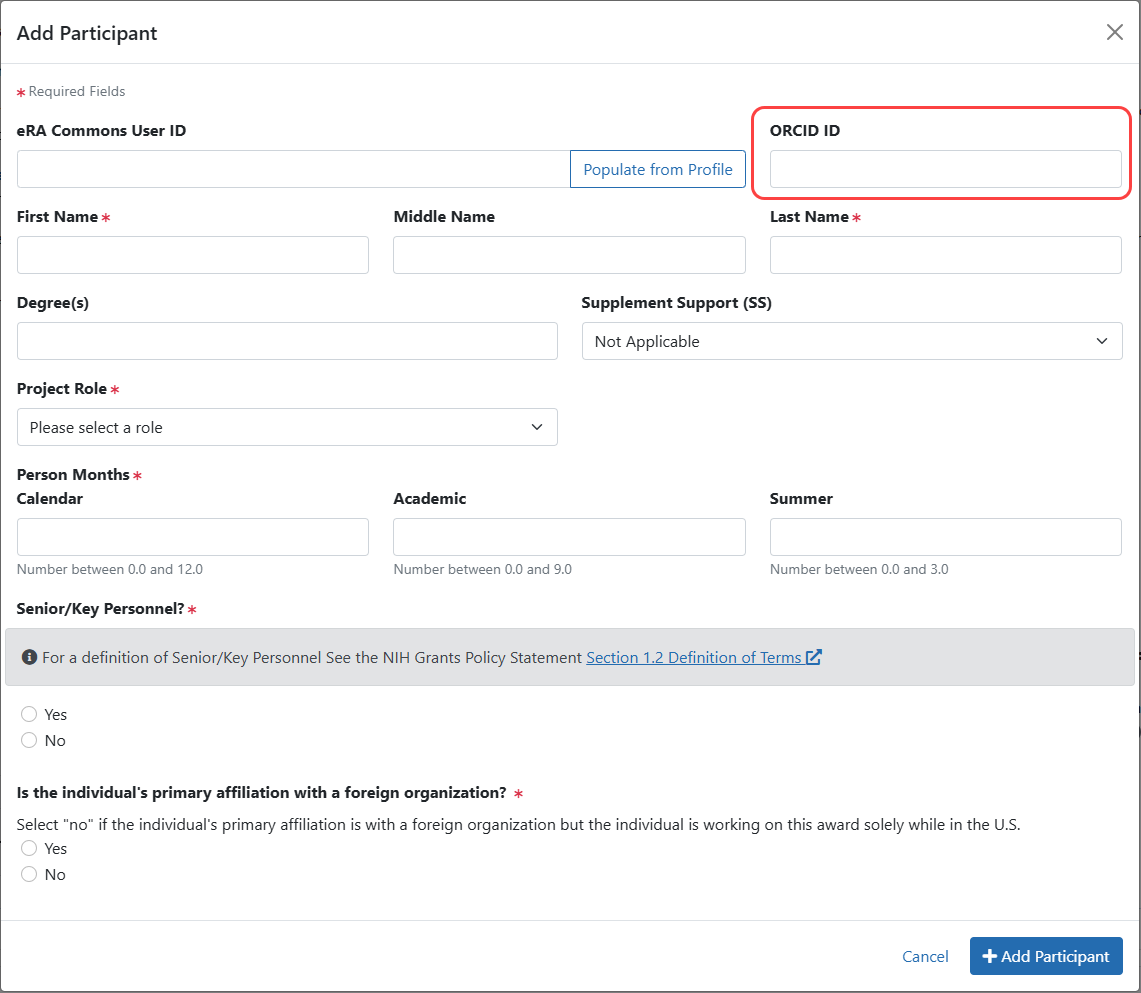
Figure 5: RPPR Participant Modal including ORCID iD
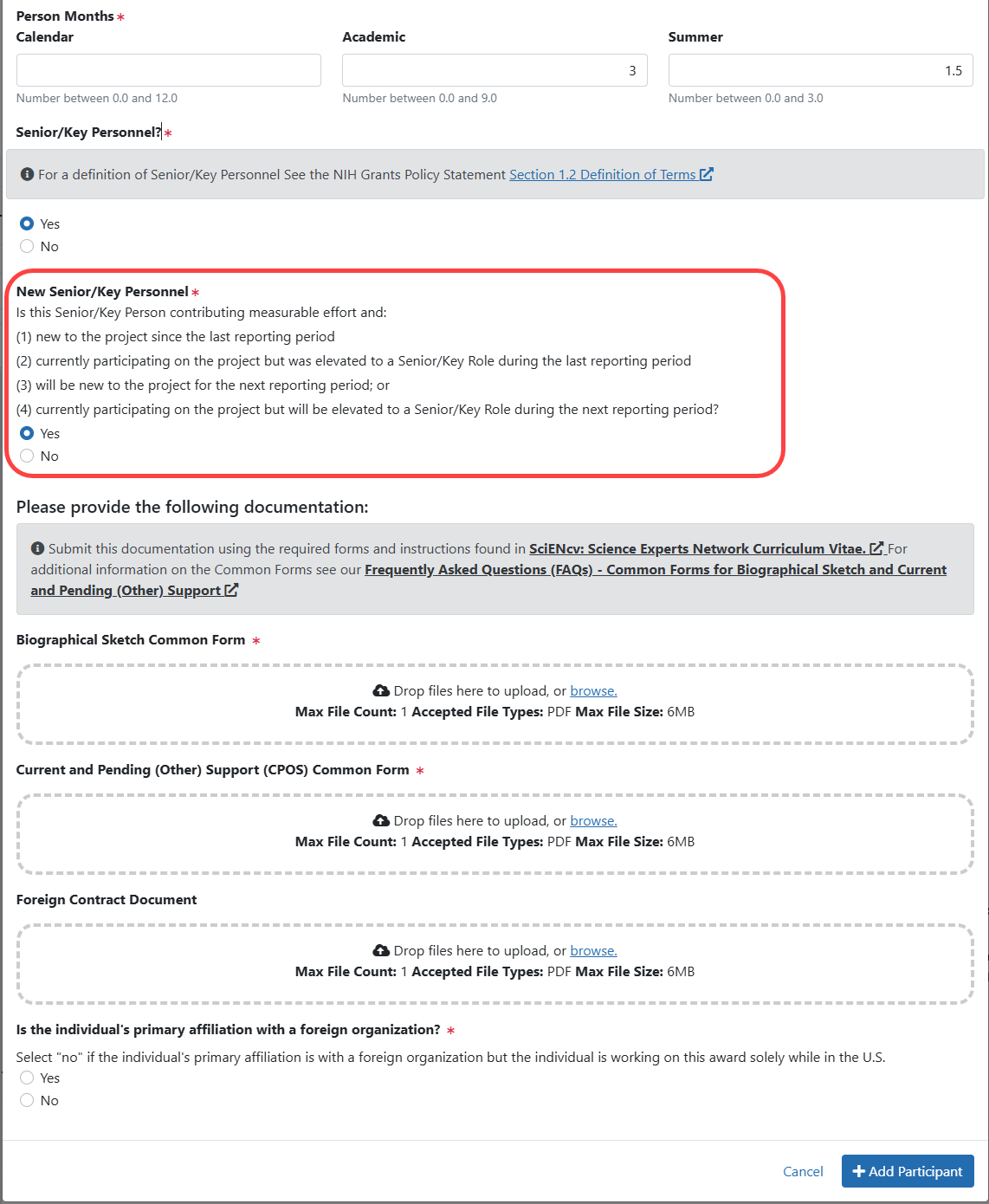
Figure 6: Participant Modal when the participant is selected as both Senior/Key Personnel and “New” (previously, Question D.2.b).
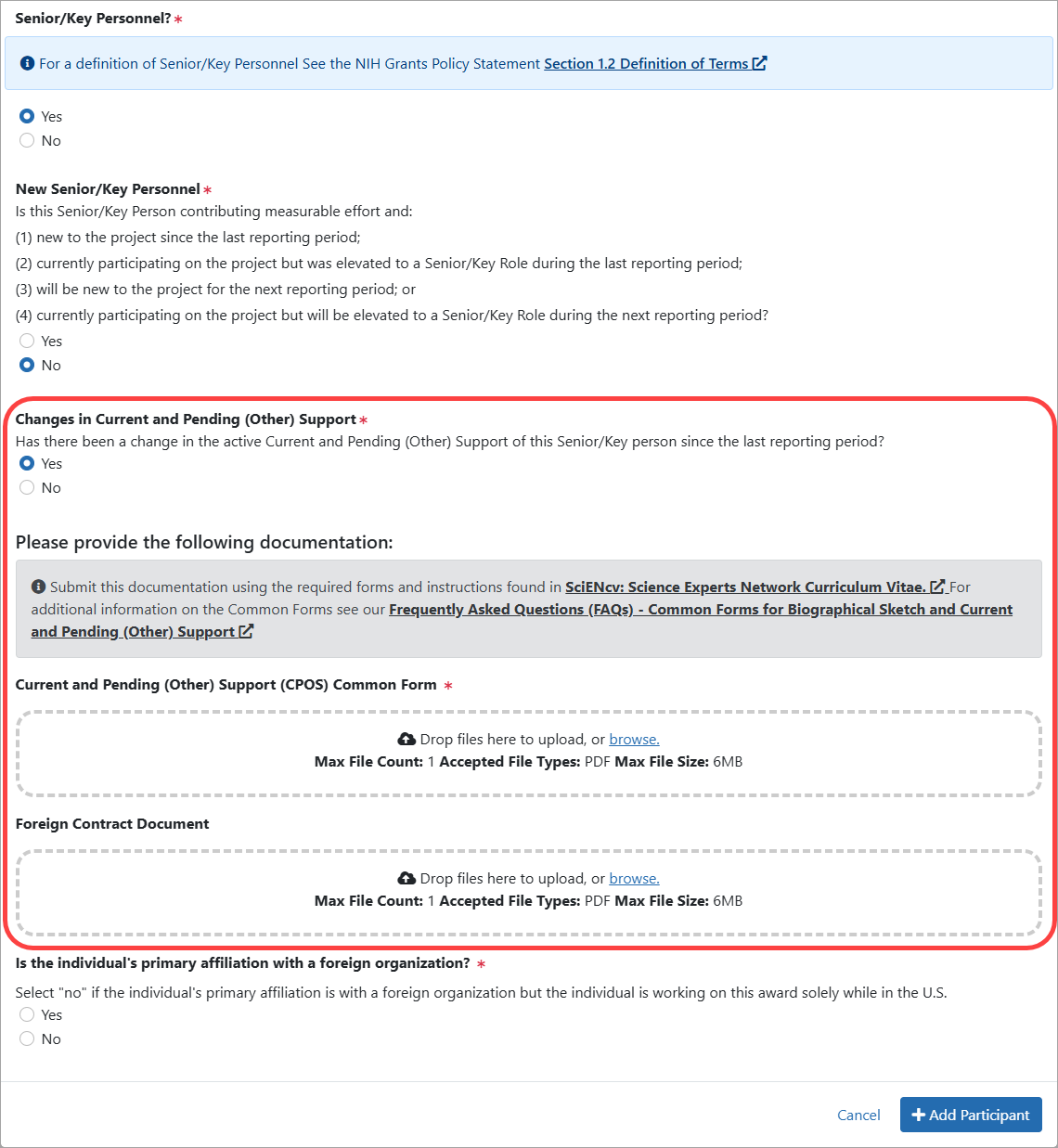
Figure 7: Participant Modal when the participant is selected as Senior/Key Personnel and a change in Current and Pending (Other) Support is indicated (previously, Question D.2.c.)
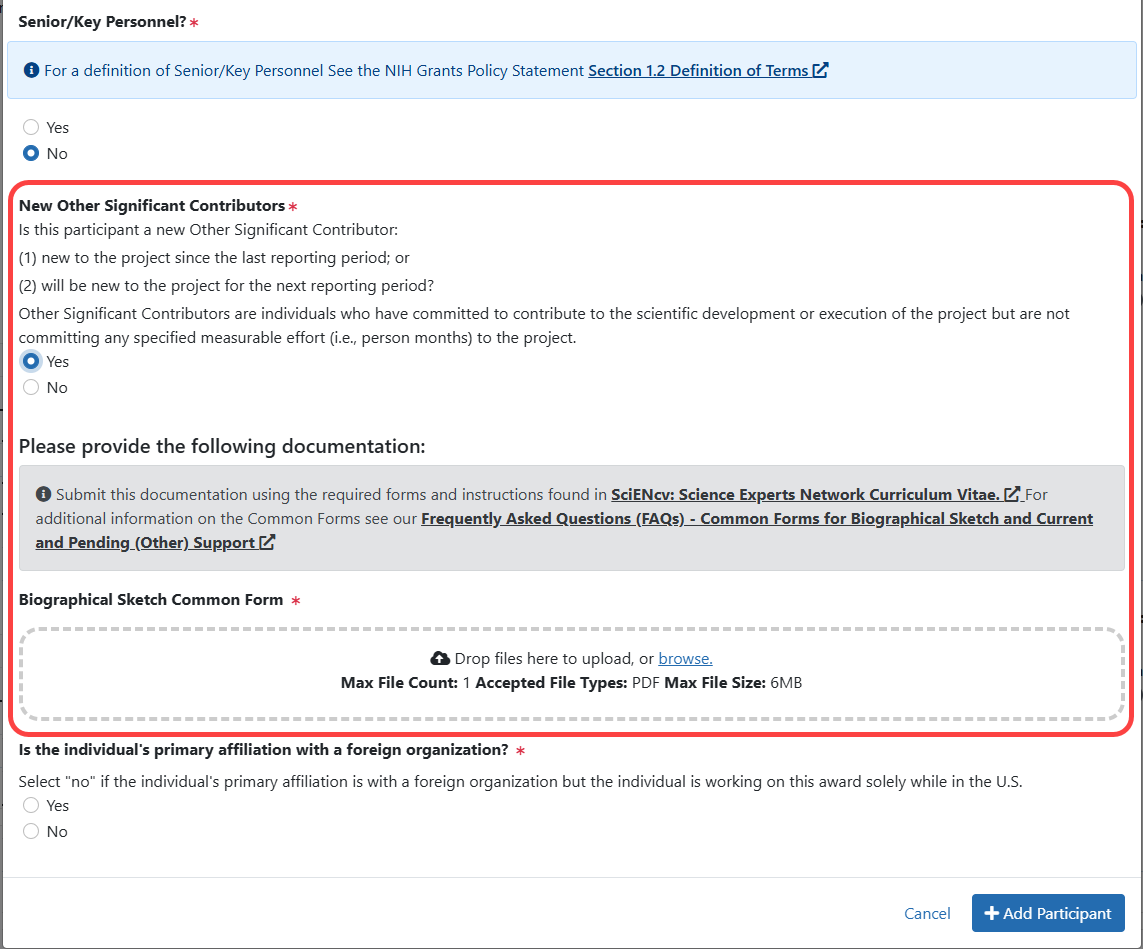
Figure 8: Participant Modal when the participant is selected as not Senior/Key Personnel and is a New Other Significant Contributor (previously, Question D.2.d).
NOTE: The participant modal will now accept values of 0.0 person months to accommodate the inclusion of New Other Significant Contributors in the list of Participants, who by definition do not contribute any measurable effort.
Resources:
- RPPR Online Help
- RPPR Instruction Guide
- Guide Notice NOT-OD-26-018: NIH’s Implementation of Common Forms for Biographical Sketch and Current and Pending (Other) Support for Due Dates on or after January 25, 2026
- Link Your ORCID iD to Your eRA Commons Account (video)
- SciENcv Workshop
- Common Forms for Biographical Sketch and Current and Pending (Other) Support (FAQs website)




 eRA Intranet
eRA Intranet