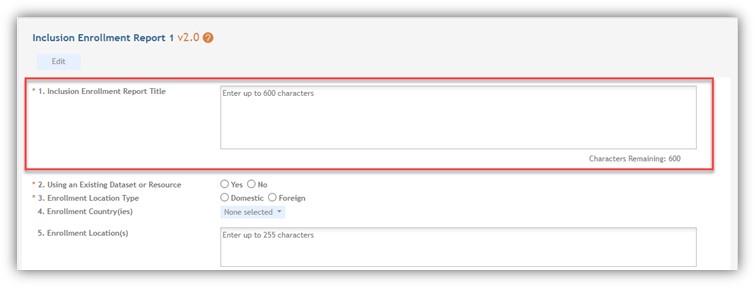
- A new Inclusion Enrollment Report title field is now available. The human subjects study title will appear by default for all existing studies and may be changed by the user. For new studies and those with a work in progress study, the title will be blank and need to be filled by the grantee. See Figure 1.
- For clinical trials, the “Brief Summary” field will no longer appear in Section 4. Information in this field prior to June 13, 2020 will continue to be available in the PDF images of applications and Research Performance Progress Reports (RPPRs) submitted prior to this change.
Question 1.4.a. “Does the study involve human subjects/participants” now has a default value of yes and cannot be changed unless the user changes their response to the question “Are Human Subjects Involved?” on the Other Projects Information form. As a study record should be completed only for research involving human participants, the “No” option is no longer available for this question. See Figure 2.

- In Section 2.8 of the Study Record: PHS Human Subjects and Clinical Trials Information screen, the language has been changed from ‘Enrollment of First Subject’ to ‘Enrolment of First Participant.’
- A new question has been added to the Study Record: PHS Human Subjects and Clinical Trials Information screen — Section 4.6 asks ‘Is this an applicable clinical trial under FDAAA.’
- For a full list of significant FORMS-F changes, see the High-level Grant Application Form Change Summary: FORMS-F PDF.
- Recipients reporting actual enrollment progress for a grant application submitted for due dates January 25, 2019 or later must provide participant-level data on sex, race, ethnicity, and age at enrollment in the Inclusion Enrollment Report of the PHS Human Subjects and Clinical Trials Information Form using HSS.
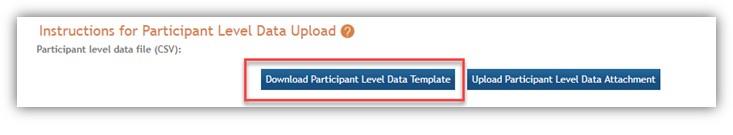
Users must use the spreadsheet template available in HSS to submit participant-level data. The column titles must not be altered, and data should be provided using the values modeled in the template. Changes to the template may result in a submission error. See Figure 3.
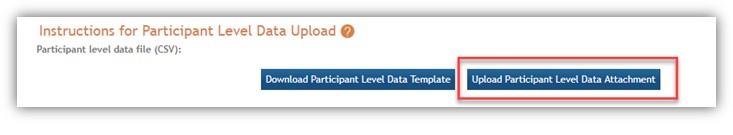
Participant-level data must be uploaded in .csv format. Users do not need to separately enter the individual numerical fields in the Actual (Cumulative) Inclusion Enrollment Report table once the .csv file is successfully uploaded. The data provided will populate the actual (cumulative) enrollment table within the system. See Figure 4.
- For more information on the requirement of individual-level participant data on sex, race, ethnicity, and age at enrollment in progress reports for research supported by NIH projects, please see NOT-OD-18-116.
- Entering Inclusion Data Using the Participant Level Data Template (video and transcript)
- Participant-level Data Template
- The eRA Human Subjects System (HSS) information page
- Information about the PHS Human Subjects and Clinical Trials Information form




 eRA Intranet
eRA Intranet