NIH policy currently requires institutions to maintain an up-to-date, written, enforced policy to identify and manage Investigator Financial Conflicts of Interest (FCOI) and to post the policy on their publicly accessible Web site. Effective November 12, 2020, NIH recipients will be required to submit their publicly assessible FCOI policy to NIH via the eRA Commons Institution Profile (IPF) Module (see IPF screen below).
This new feature will apply to all NIH applicants and/or recipients, with the exception of Phase I Small Business Innovative Research (SBIR) or Small Business Technology Transfer (STTR) applicants and/or recipients. (See NIH GPS Section 4.1.10 Financial Conflict of Interest for more information). This new feature does not change the current requirements.
While the requirement goes into effect in November, NIH recognizes that recipients will need time to modify their internal systems in order to comply. Therefore, applicants and recipients have until December 1, 2020 to comply with this requirement. The new feature within the IPF screen becomes available on November 12, 2020.
Note that noncompliance with this requirement as of December 1, 2020 may cause NIH to delay the issuance of awards, impose specific award conditions or take other enforcement actions.
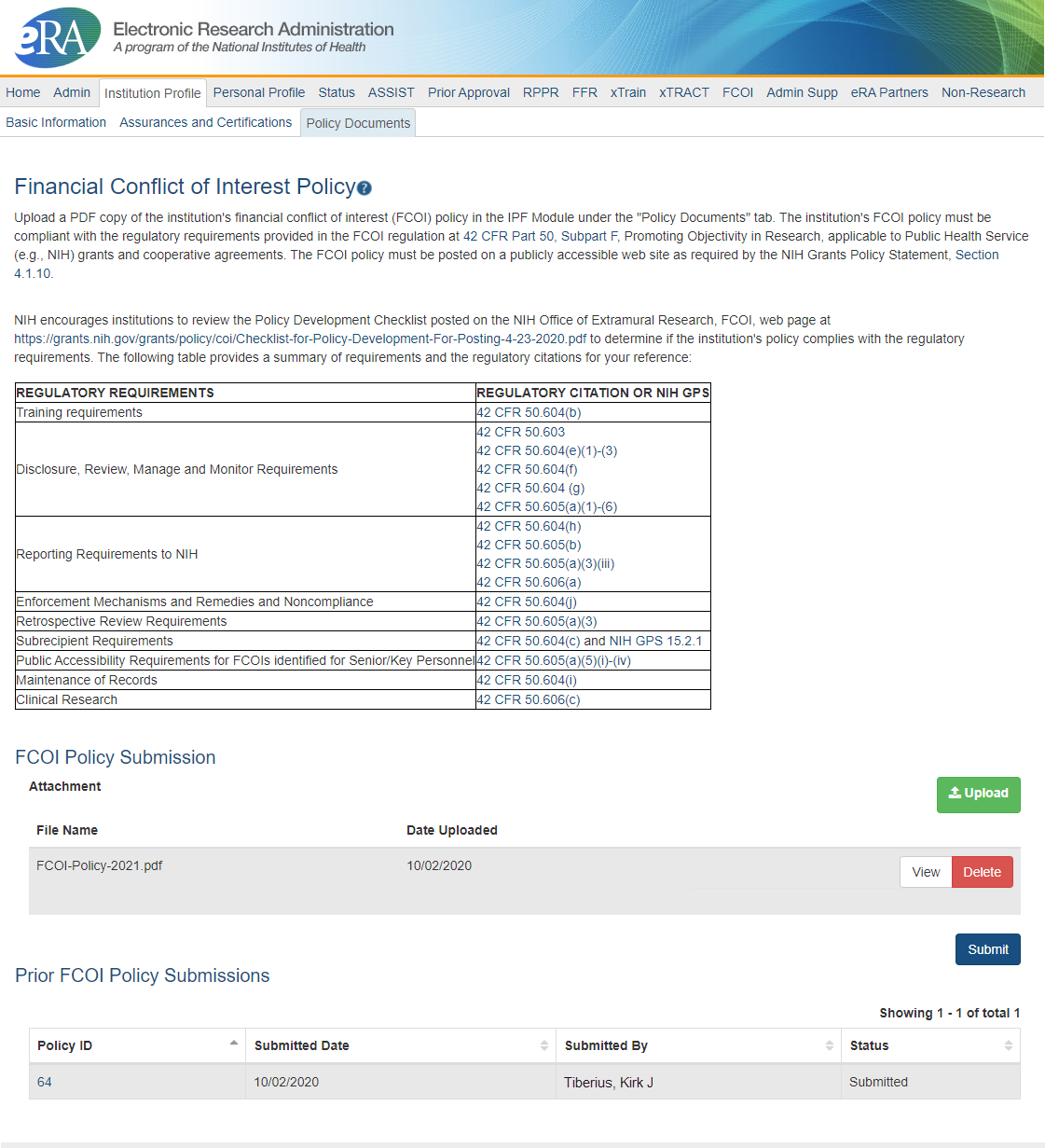
Figure 1: Financial Conflict of Interest Policy submission screen in the IPF module
A PDF of the FCOI policy must be uploaded to the IPF by the institutional signing official (SO) under a new tab labeled “Policy Documents.” The information is provided on an institutional level as part of an institution’s IPF, rather than on a grant-specific level, so it is not necessary to submit the FCOI policy with each grant application.
NIH will review the submitted FCOI policies to ensure compliance.
Future Enhancements
NIH plans to make future enhancements to the IPF Module which will require institutions to:
- Provide the URL of the FCOI policy
- Complete an annual institutional FCOI certification of compliance with all identified regulatory requirements
As part of the annual certification, institutions will be required to provide an updated URL and a copy of the revised policy if changes have been made since the last submission. NIH will provide reminder notifications to the SO when the annual certification is required.
Resources
- NIH Guide Notice NOT-OD-21-002
- For an overview of the IPF, see Overview of Institutional Profile (IPF)
- NIH’s FCOI Web page: https://grants.nih.gov/grants/policy/coi/index.htm
- FCOI Compliance email: FCOICompliance@mail.nih.gov
electronic Research Administration (eRA)
NIH Office of Extramural Research
Questions? Please contact the eRA Service Desk. Check out self-help resources on the Help page before submitting an online ticket; or call Toll-free: 1-866-504-9552, Phone: 301-402-7469. The eRA Service Desk hours are Mon-Fri, 7 a.m. to 8 p.m. ET.
Help us improve our communications; send your suggestions and feedback to eRACommunications@mail.nih.gov or call 301-435-8185.
To read other recent articles and messages, please visit our Latest News page at https://era.nih.gov/news
We are sorry if you receive duplicates. This notification was sent to multiple distribution lists. To subscribe to or unsubscribe from our listservs, please visit https://era.nih.gov/about-era/get-connected.htm.




 eRA Intranet
eRA Intranet