New features and fixes added to the Commons module are listed below:
Year: 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2022 | 2023
2023
March 16, 2023
eRA Enhancements: Redesigned xTrain Module in eRA Commons Coming on March 30
A newly redesigned xTrain module in eRA Commons is scheduled to be rolled out on Thursday, March 30, following a collaboration with an external user group. The new xTrain, with revamped screens and streamlined processes, is designed to be more user-friendly and task oriented. In addition, xTrain will adopt the new visual appearance being adopted by other eRA modules, as part of a required technology upgrade.
As a reminder, xTrain is used by program directors/principal investigators (PD/PIs), university administrators, and trainees to electronically prepare and submit PHS 2271 Statement of Appointment Forms and PHS 416-7 Termination Notices associated with institutional research training grants, institutional career development awards, individual fellowships, and research education awards.
The release will accommodate a clarification on signatures for trainees (see section towards the end of the message below). Read details about this change and a clarification of organizational responsibilities for external users in NIH Guide Notice NOT-OD-23-094.
Although the entire module will be updated, only a sampling of new screens is shown below. For complete screenshots and steps, see the xTrain online help following the release.
Awarded Grants Screen
The Awarded Grants screen is the first screen PIs, sponsors, and their delegates see upon login. Note that business officials, signing officials, and trainees see slightly different screens tailored for their task flows. The Awarded Grants screen lists grants awarded to the current user as well as grants for which the current user is a sponsor. Users can use the Quick Filters section, a set of toggles that narrow down the grants shown. Actions that were formerly under the Action column, such as the option to go to the training roster, appear under the three-dot ellipsis menu; see Figure 1.
A set of new tools at the top of tables in xTrain lets you type text to quickly filter results, print or download results, choose results shown per page, and navigate through pages of results; see Figure 1. For information on using new table tools, see Standard Tools for Tables.
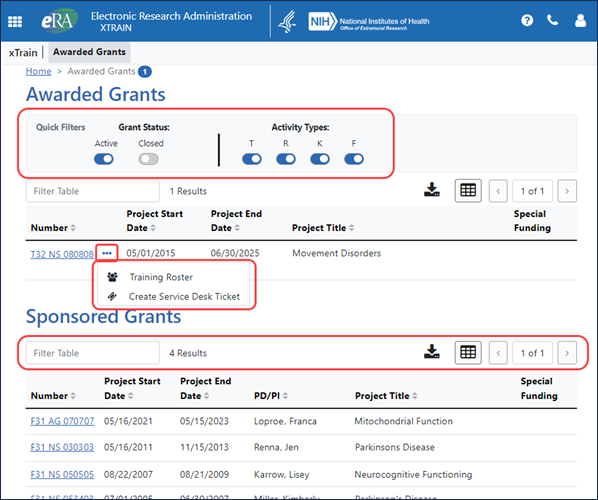
Figure 1: Awarded Grants screen showing Quick Filters to narrow down grants shown, the three-dot ellipsis menu displaying actions for a grant, and new table tools
Trainee Roster Screen – Overview Section
The top of the Trainee Roster screen shows grant information and trainee roster information by grant year and includes graphs of awarded and accepted months for pre-doc, post-doc, and short term. For a particular grant year, users can click an Appointments – Start New button to search for a trainee and start a new appointment form. All actions available for a grant reside under the three-dot ellipsis menu next to the grant number.
The See Slots link for each year will show slots awarded and accepted for each of the three categories of appointments (pre-doc, post-doc, and short term); see Figure 2. More complete slot data is available from the Appointment Form screen; see Figure 5.
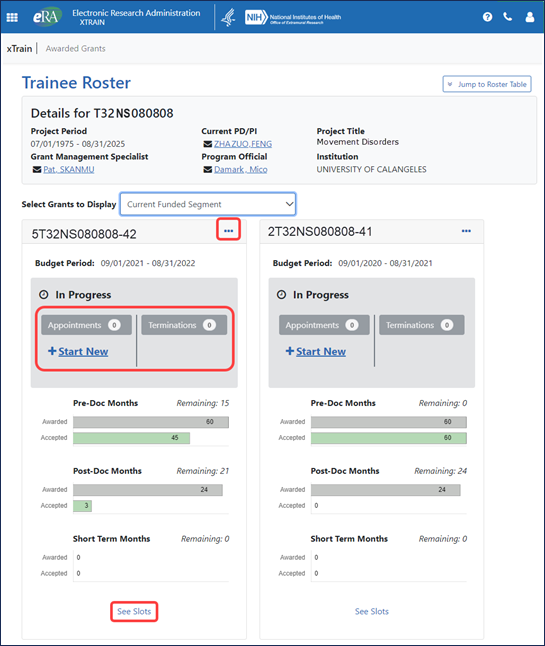
Figure 2: Top part of the Trainee Roster screen, which graphically displays current trainee months, as well as a three-dot ellipsis for performing actions, number of Appointments and Terminations, a Start New appointment link, and a See Slots link for each grant
Trainee Roster Screen – List of Trainees Section
At the bottom of the Trainee Roster screen, which users can quickly access using the Jump to Roster Table button, users see the list of trainees. Users can click the three-dot ellipsis menu for a trainee to see available actions such as view the appointment form, view routing history, initiate or view a termination notice (not shown), amend an appointment, or create a service desk ticket that is automatically populated with the relevant information. Underlined and linked trainee names represent forms that are a work in progress by the trainee, but accessible to be recalled by the PI; see Figure 3.
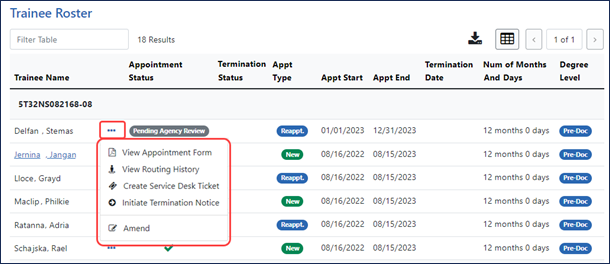
Figure 3: Bottom of the Trainee Roster screen, which lists trainees as well as the three-dot ellipsis menu displaying action items
Appointment Form Screen
On the Appointment Form screen, accessible when creating or amending in-progress appointments, users can specify the details of a trainee as well as the trainee’s appointment, save the form as a draft, and eventually submit the form to the agency; see Figure 4.
Not shown is a similar screen for terminations, titled Termination Notice, which can be accessed for trainees who are eligible to have their appointments terminated.
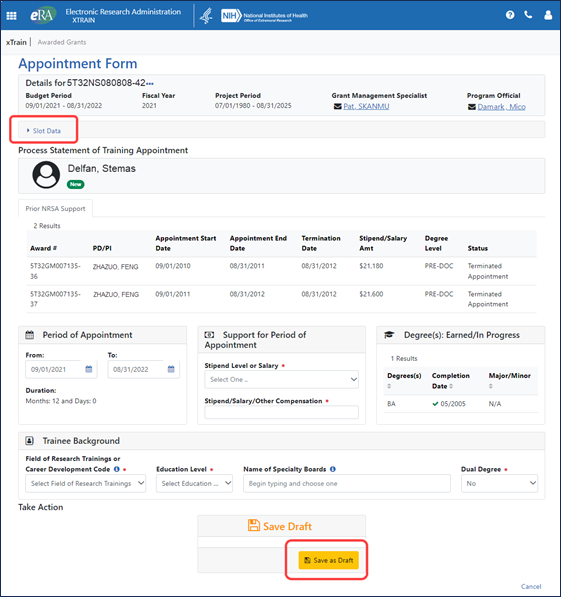
Figure 4: Appointment Form screen, where users can specify the details of a trainee’s appointment, also displaying the Slot Data link and Save as Draft button
To quickly check how many slots are accepted, awarded, and remaining, the user can click the Slot Data link, shown outlined in red above, which expands the section; see Figure 5.
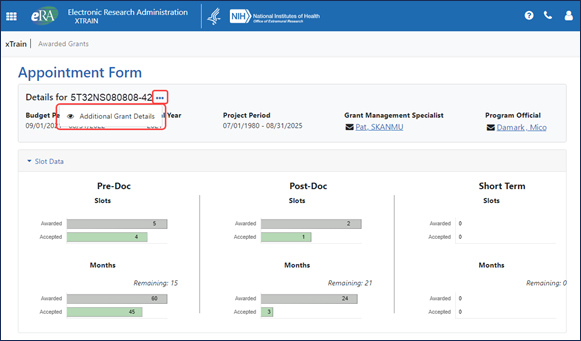
Figure 5: Slots and months for one grant year broken down into accepted, awarded, and remaining for pre-doc, post-doc, and short term
A Process Change for Deletion of Forms
Deletion of Forms
With this release, the process for deletion of forms will change. Agency staff will return the form to the organization to delete, instead of deleting the records on their own.
Clarifications on Signatures for Trainees and of Organizational Responsibilities for External Users
Trainee Signature to be Required on Reappointments and Amendments
Trainees will be required to sign Reappointments and Amendments, just as they do for Initial Appointments. In cases where this is not possible due to a trainee’s unavailability, the program director/principal investigator (PD/PI) will add a comment to explain why the trainee could not sign.
Clarification of Organizational Responsibilities for Type 6 and Type 7 Actions
NIH has issued a clarification of an organization’s responsibilities for Change of Recipient Organizations (Type 7) and Successor-in-Interest/Name Change (Type 6) actions (details in guide notice below).
2022
October 25, 2022
eRA Enhancements: New Status Screens for Signing Officials to Be Released October 27
We are pleased to inform you that the eRA Commons Status module for signing officials (SOs) is moving to the new visual appearance being adopted by other eRA modules, as part of a required technology upgrade. New screens will be rolled out during a software release on Thursday, October 27, 2022.
As a reminder, Status screens are used by applicants and recipients to find the current status of an application or award and check on upcoming actions they need to take. They are also used for closeout and change of institution activities. The Status module appears different for signing officials than for principal investigators. Only the SO view of Status is being updated with this release; the Status screens seen by principal investigators remain the same.
New Search Screen
The new search screen contains all the types of searches under the Search Type dropdown instead of listing them at the left. When you select a particular search, the screen dynamically changes or redirects you to a new screen that reflects the search.
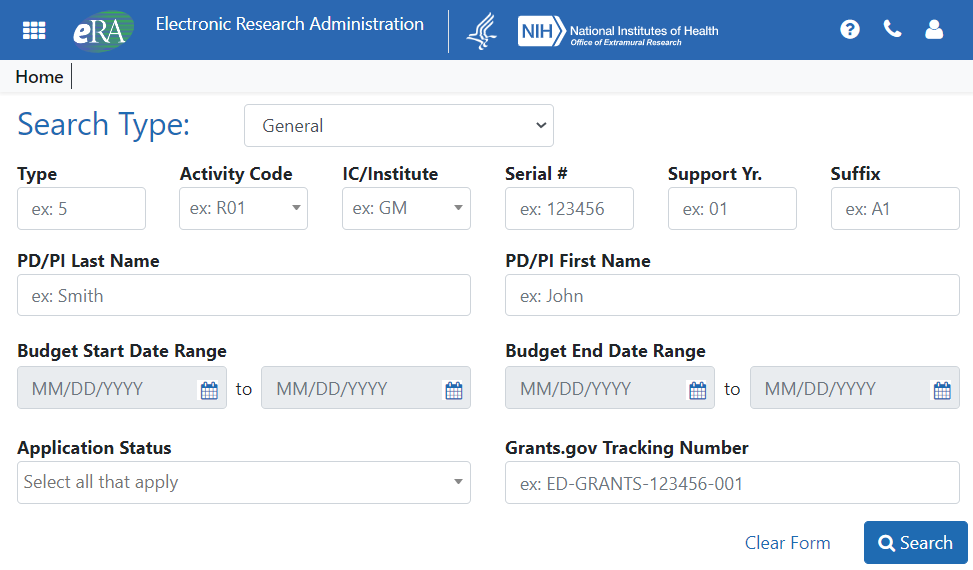
Figure 1: New Status search screen for SOs
For instance, if you select Recent/Pending eSubmissions from the Search Type dropdown (see Figure 2), you are redirected to a separate search screen designed for that purpose. However, if you select Relinquishing Statements, you remain on the Status search screen, but search criteria related to relinquishing statement status appear.
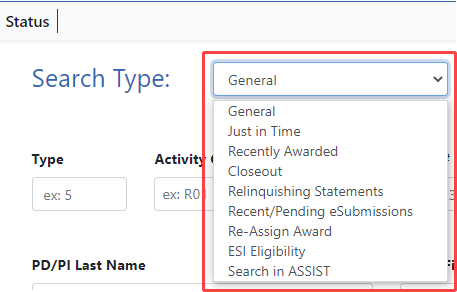
Figure 2: Status Search Type dropdown for SOs
New Three-Dot Ellipsis Icon for Performing Actions
Instead of buttons in an Actions column, you now click the three-dot ellipsis icon for an application or grant record and choose actions from the menu that appears; see Figure 3.
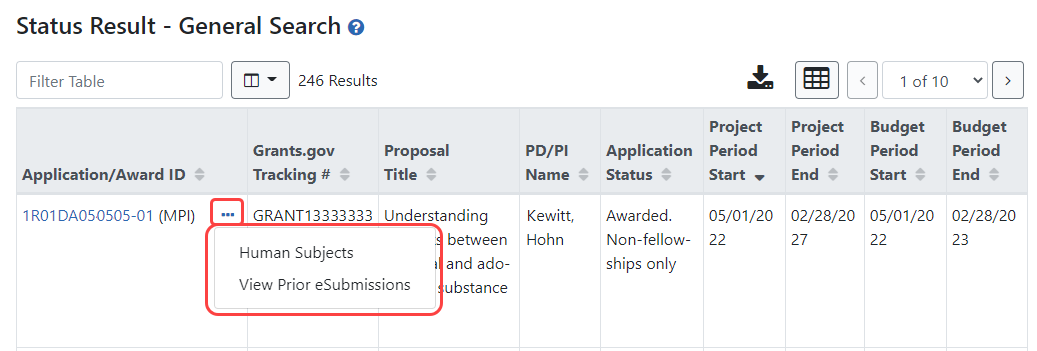
Figure 3: New Status search results screen for SOs shows actions under three-dot ellipsis menu
Enhanced Table Tools
New table tools let you filter based on text you enter, choose visible columns, download or print, choose how many results appear per page, and navigate through the pages of search results. See Figure 4. Also see Navigating and Using the UI in eRA Modules in the eRA Commons online help for descriptions of table tools.
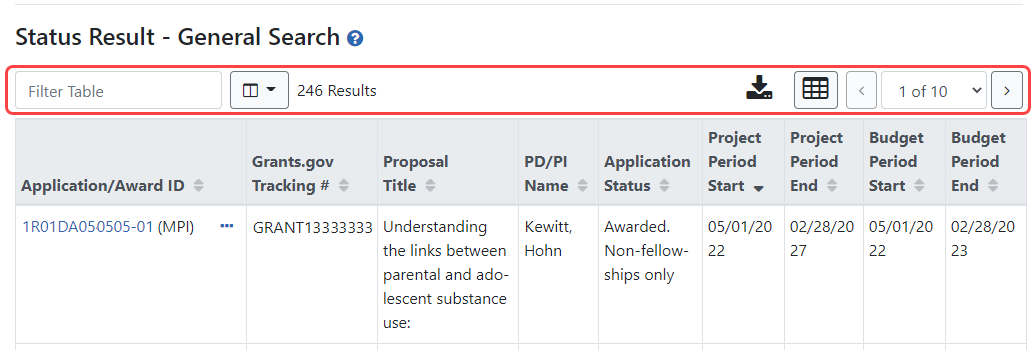
Figure 4: New table tools for working with tables in Status search results
Relinquishing Statement Screens Updated
The Relinquishing Statement screens, used to effect a change of institution for an award, have been updated with the new look and feel. You can search for awards that are eligible to be relinquished to other institutions, awards that have had a relinquishing statement in progress at your institution, and awards that are in the progress of being relinquished to your institution.
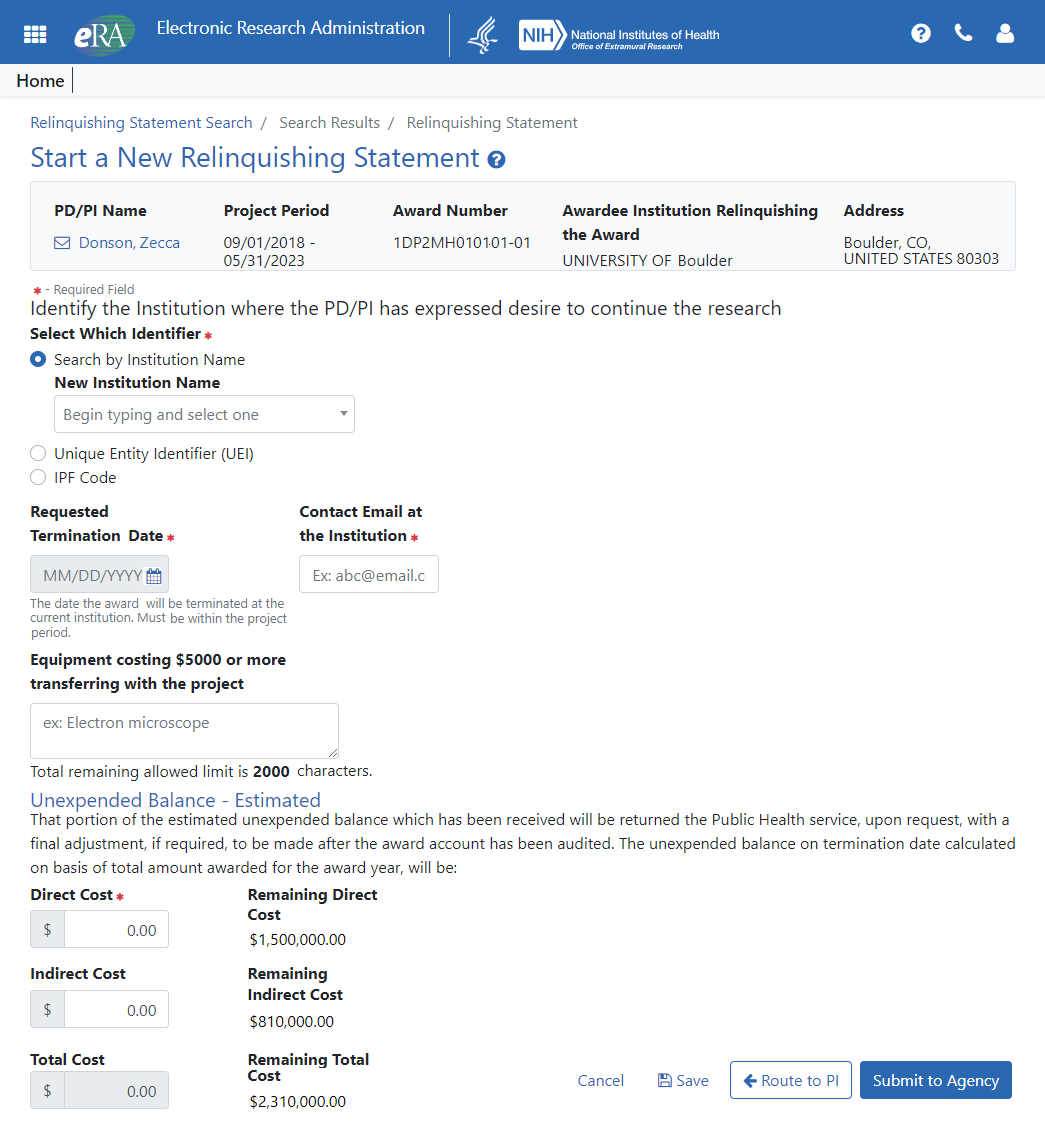
Figure 5: New screen for SOs to start a new relinquishing statement
Closeout Status Screens Updated
The Closeout Status screen and related screens, such as those for processing a final invention statement, have been updated to the new look; see Figure 6.
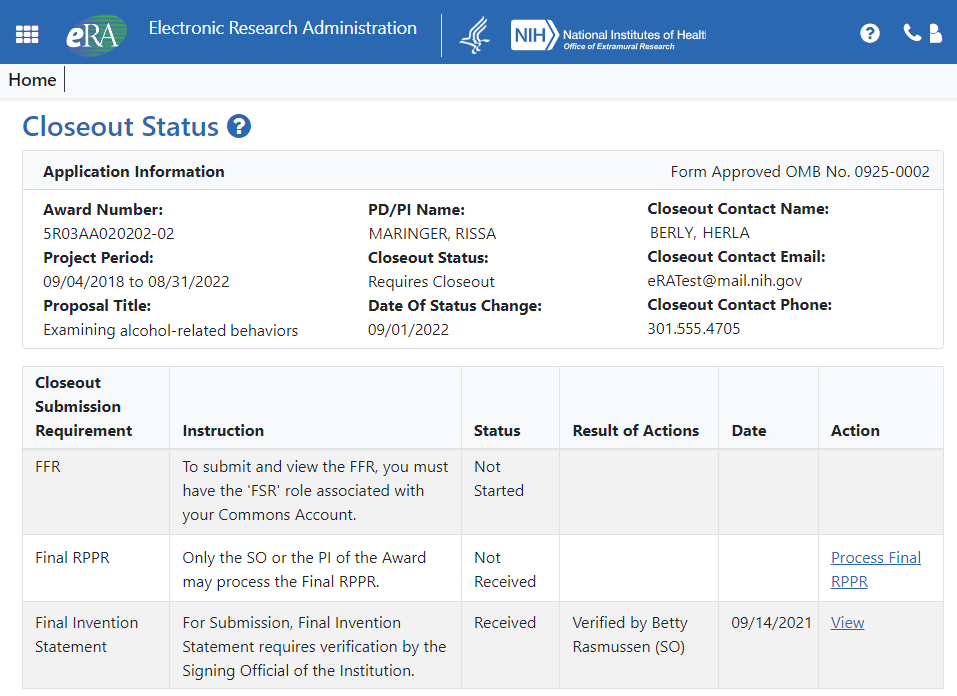
Figure 6: New Closeout Status screen for SOs
Highlights of New Changes
All screens will adopt the new standard features being incorporated in other eRA modules, such as:
- Apps menu icon — The apps menu, located in the upper left of the new blue header, shows a dropdown menu of other eRA modules available to the user.

- Header and Footer — An elegant header and footer take up less space, leaving more real estate for the functionality of the module.
- Action items — A three dot ellipsis icon, when clicked, displays a dropdown menu of action items. This icon replaces the action column. See Figure 3.
- Enhanced Table Tools — A set of tools enhances the ability to work with table data, with easier filtering and page navigation; see Figure 4.
- Collapsible Information in Search Results (Relinquishing Statements) — Search results for relinquishing statements now utilize a collapsible view. You can use the arrow, outlined in red below, to expand and collapse additional information about a relinquishing statement. Perform actions on the item by clicking its three-dot ellipsis menu, also outlined below. See Figure 7. Following the release, see Managing Relinquishing Statements in online help.
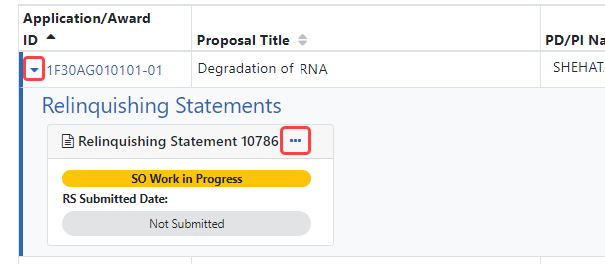
Figure 7: Search results for relinquishing statements, showing the arrow used to expand/collapse additional information, and the three-dot ellipsis menu used to perform actions on the relinquishing statement
For an explanation of all the new look and feel features, see Navigating and Using the UI in eRA Modules in the eRA Commons online help.
June 23, 2022
eRA Reminder: Updated Research Performance Progress Report (RPPR) Screens Released Today
We would like to remind you that today, June 23, 2022, all RPPR screens have been updated to a new visual appearance, with minimal functional changes made to accommodate FORMS-G and new unique entity identifiers (UEI) as a replacement for DUNS numbers. An updated RPPR Instruction Guide that aligns with the new screens, FORMS-G, and UEIs has also been released today. See details in the prior messages sent regarding the RPPR update, which are below.
June 23, 2022
eRA Reminder: New Look for FCOI Module to Be Released in eRA Commons on June 30
We are pleased to inform you that the FCOI Module in eRA Commons is moving to the new visual appearance being adopted by other eRA modules, as a result of a required technology upgrade. The refreshed screens are being rolled out Thursday, June 30, 2022. The functionality and content remain the same.
As a reminder, FCOI screens are used to manage the Financial Conflict of Interest (FCOI) reporting process for a particular institution by reporting the existence of identified FCOIs involving principal investigators to the granting agency. See the Overview of Financial Conflict of Interest for more information.
Highlights of New Changes
The screens will adopt the new standard features being incorporated in eRA modules, such as:
- Apps menu icon — The apps menu, located in the upper left of the new blue header, shows a dropdown menu of other eRA modules available to the user.

- Buttons — If multiple buttons exist in a row, the primary button will be prominent and the secondary ones will either be outlined or have no border, to help highlight the options that are more likely to be used.

- Header and Footer — An elegant header and footer take up less space, leaving more real estate for the functionality of the module.
- Action items — A three dot ellipsis icon, when clicked, displays a dropdown menu of action items. This icon replaces the action column. See Figure 1.
- Enhanced Table Tools — A set of tools enhances the ability to work with table data, with easier filtering and page navigation.
For an explanation of all the new look and feel features, please see Navigating and Using the UI in eRA Modules in the eRA Commons online help.
New Three-Dot Ellipsis Icon for Performing Actions
Instead of buttons in an Actions column, you now click the three-dot ellipsis icon for a record and choose actions from the menu that appears; see Figure 1.
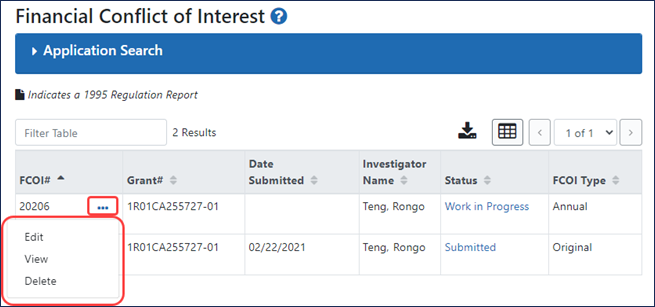
Figure 1: Search results, showing FCOI records with a three-dot ellipsis menu.
Drag and Drop File Attachment
On FCOI record details screens, you can now drag and drop files in addition to browsing to a file on your drive; see Figure 2.
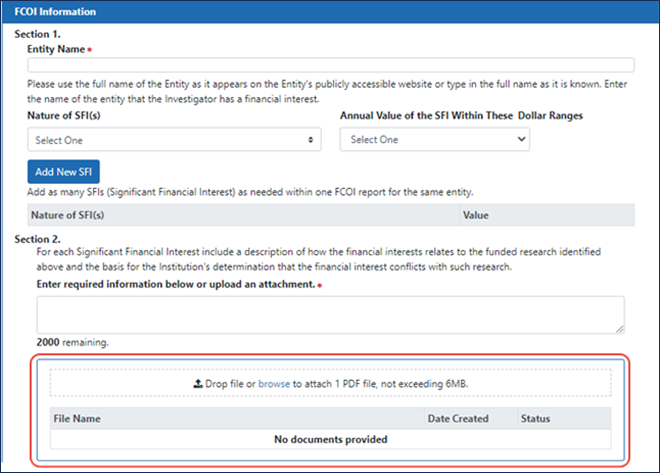
Figure 2: New FCOI details screen with drag and drop area.
May 23, 2022
eRA Information: UEI Field Will be Added to Institutional Profile in eRA Commons Tomorrow, May 12
The Institutional Profile screen in eRA Commons will accommodate the Unique Identity Identifier (UEI) following a release May 12, 2022.
As you are aware, as of April 4, 2022, the UEI replaced the Dun & Bradstreet (DUNS) number as the official identifier required for an organization/institution to apply for and receive federal funding from NIH and other federal agencies (See NIH Guide Notice NOT-OD-21-170). The UEI, a 12-character alpha numeric identifier, is issued as part of the System for Award Management (SAM) registration process. For applications due on or after January 25, 2022, applicant organizations must have a UEI at the time of application submission.
Highlights
- The Primary DUNS field will be renamed as the Primary UEI/DUNS field. While DUNS is no longer being used, we have retained the nomenclature to help ease the transition from DUNS to UEI for users.
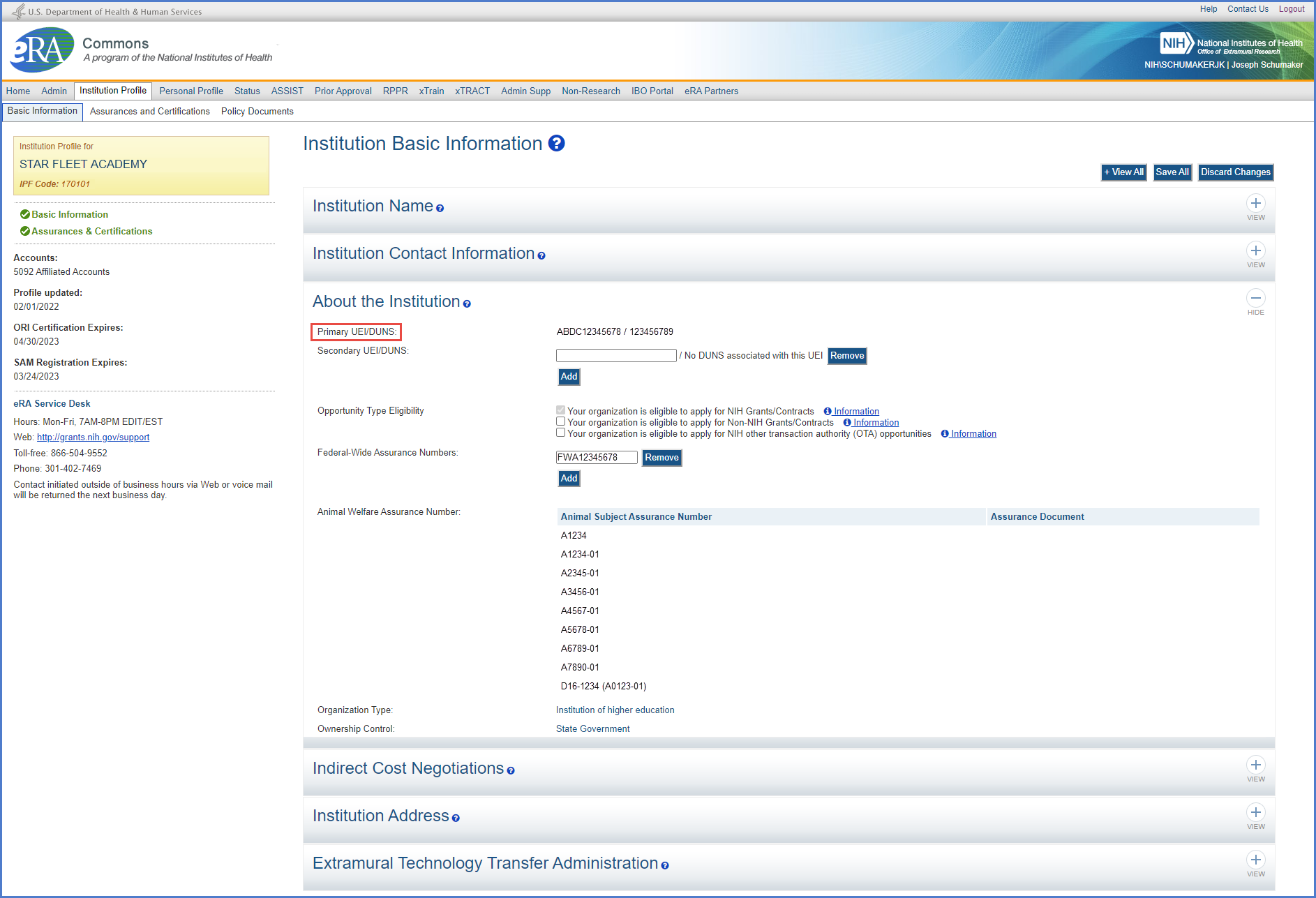
Figure 1: The Primary UEI/DUNS field in the Institution Profile in eRA Commons
- If the UEI has been provided at registration, the UEI will be automatically populated.
- If the user has not provided a UEI with the registration, this is a fillable field for the user to provide the primary UEI. If there is a corresponding DUNS in the system, the DUNS will be automatically displayed.
Note: If the UEI is entered with a missing letter or number, a message will alert the user who will need to reenter the UEI.
- A secondary UEI can be added by clicking the Add button next to the Secondary UEI/DUNS field.
- The screen also has a section to ‘View Primary/Secondary DUNS for your organization prior to April 2022.’ This is for users to view their organization’s primary and secondary DUNS that existed before the April transition to UEI.
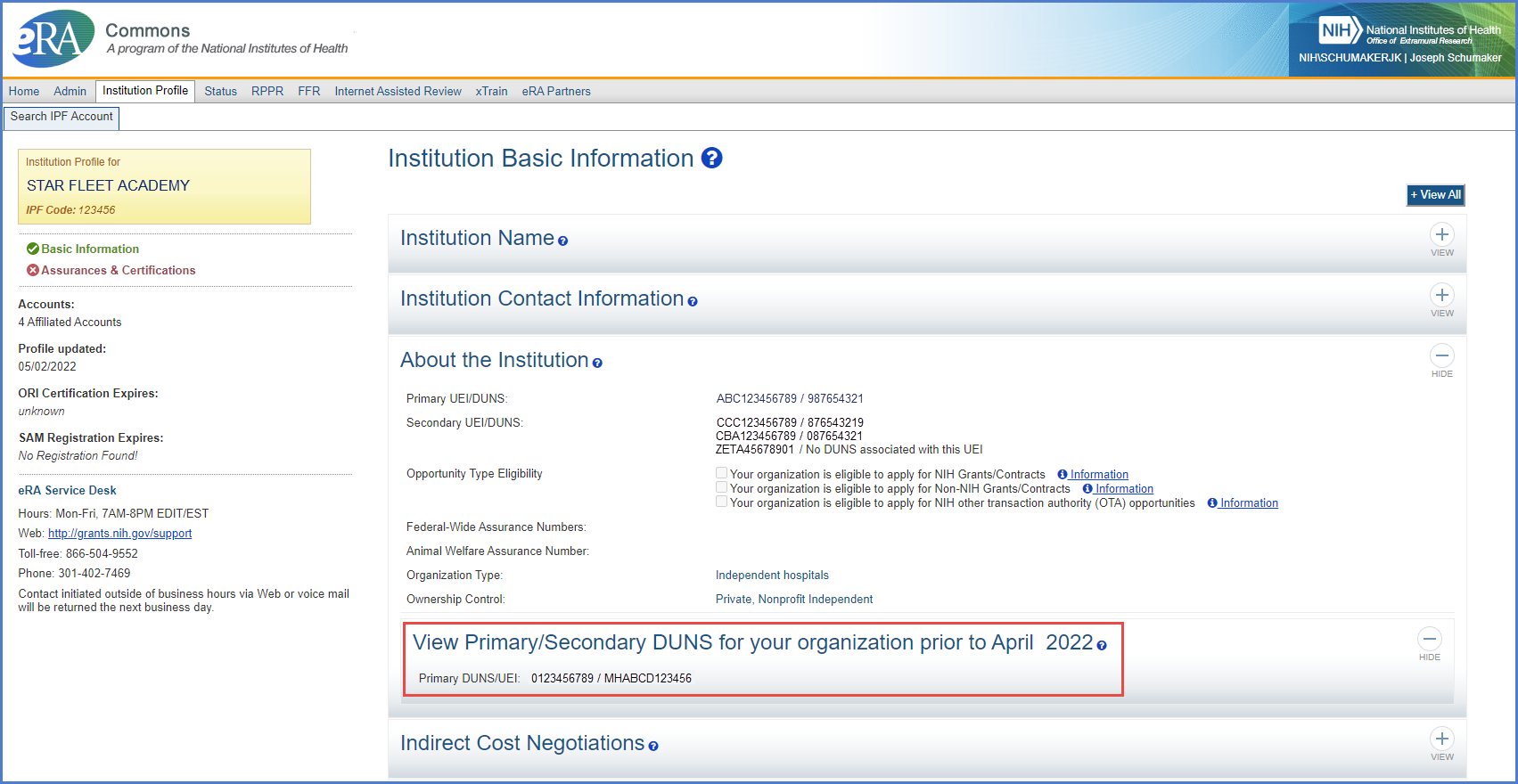
Figure 2: The ‘View Primary/Secondary DUNS for your organization prior to April 2022’ section in the Institution Profile in eRA Commons
May 16, 2022
eRA Enhancements: New Look for Delegations in eRA Commons Released Monday, May 16
We are pleased to inform you that the Delegations screen in eRA Commons is moving to the new visual appearance being adopted by other eRA modules, as a result of a required technology upgrade that enhances the security and stability of the module. The refreshed screens are being rolled out Monday, May 16, 2022. The functionality and content remain the same.
As a reminder, Delegations screens are used to either delegate your own authority to another user, or to delegate authority for one user to another user. Not all authority can be delegated, and delegation works within the context of roles. See the Delegations topic in eRA Commons online help for more information.
As a result of the new look and feel, actions are performed differently, screens are organized differently, and the technology allows for some time-saving field functionality, shown below.
Actions Moved to Three-Dot Ellipsis Menu
The actions, such as Edit Delegations, Delete Delegations, and Select, which formerly appeared as buttons in the Actions column, are now found under the three-dot ellipsis menu. See Figures 1 and 3 (below).
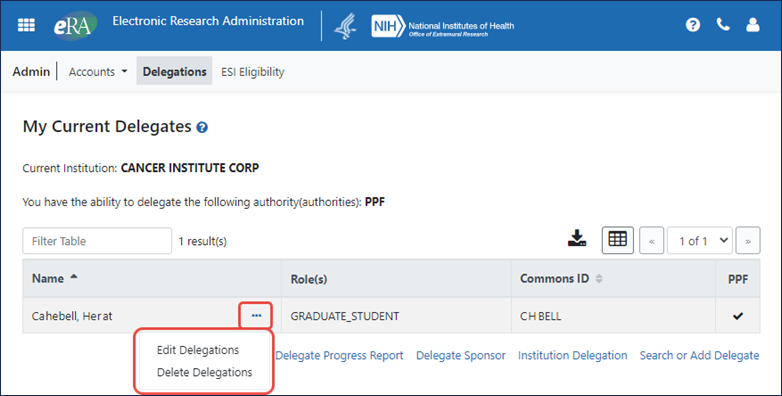
Figure 1: The actions, shown on the main delegation screen, are under a three-dot ellipsis menu.
Institution Delegation Screen Split into Two Tabs
The Institution Delegation screen formerly displayed two tables of information, one to show current delegates, and the other to configure new delegations. Those two tables are now shown on separate tabs. Use the Current Delegates tab to view or revoke authority. Use the Candidates for Delegation tab to search for and configure new delegations. See Figure 2.
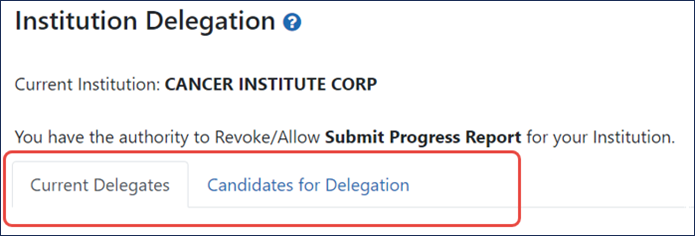
Figure 2: Institution Delegation screen shows data on two tabs.
Type Ahead Technology Implemented
Type ahead functionality has been implemented where appropriate in text boxes, allowing users to type a few letters of the name they are looking for, whereupon they are presented with a list of matching names. See Figure 3.
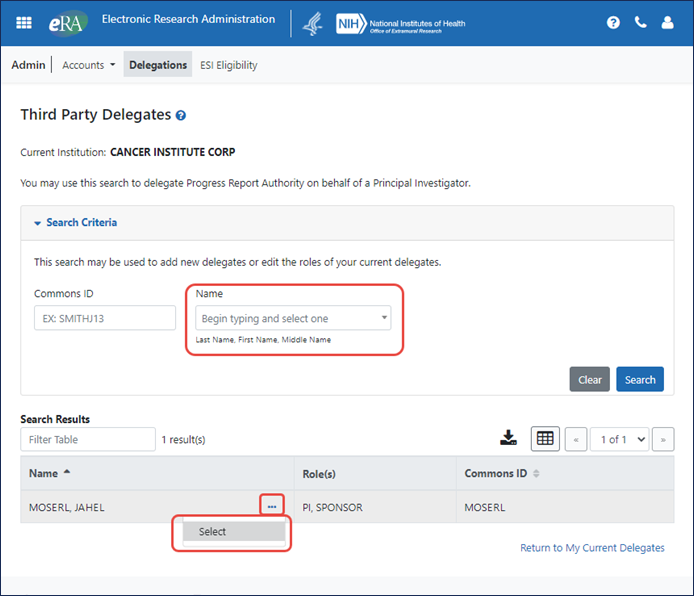
Figure 3: Third Party Delegates screen showing type-ahead field and three-dot ellipsis menu with actions.
Toggles Replace Checkboxes
Instead of checkboxes, toggles are used to select authorities to delegate. See Figure 4.
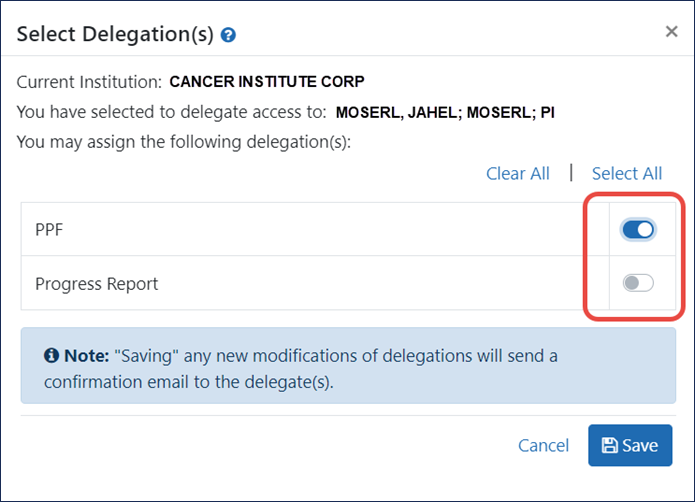
Figure 4: Select Delegation(s) popup showing toggles to select authorities to delegate.
March 4, 2022
eRA Enhancements: New Look Coming March 7 to Prior Approval Module in eRA Commons
We are pleased to inform you that the Prior Approval Module in eRA Commons is moving to the new visual appearance being adopted by other eRA modules, as a result of a required technology upgrade. The refreshed screens will be rolled out on Monday, March 7, 2022. The release will take place with no downtime or impact on users. The functionality and content will remain the same with a few updates shown below.
As a reminder, these screens are used to seek prior approval (if needed) for certain activities from the funding agency. These include requests for a no-cost extension, for a carryover of funds, for a change in Program Director/Principal Investigator (PD/PI) and for withdrawal of an application. See the Seek Prior Approval (if Needed) webpage for the circumstances and conditions under which these requests can be made.
Highlights of New Changes
The screens will adopt the new standard features being incorporated in eRA modules, such as:
- Apps menu icon — The apps menu, located in the upper left of the new blue header, shows a dropdown menu of other eRA modules available to the user.

- Buttons — If multiple buttons exist in a row, the primary button will be prominent and the secondary ones will either be outlined or have no border, to help highlight the options that are more likely to be used.

- Header and Footer — An elegant header and footer take up less space, leaving more real estate for the functionality of the module.
- Action items — A three dot ellipsis icon, when clicked, displays a dropdown menu of action items. This icon replaces the action column. See Figure 3.
- Enhanced Table Tools — A set of tools enhances the ability to work with table data, with easier filtering and page navigation.
For an explanation of all the new look and feel features, please see Navigating and Using the UI in eRA Modules in the eRA Commons online help.
In Progress Requests Show by Default
Instead of clicking a button to show in progress requests, these requests are now shown by default. The requests you see depend on your role; signing officials (SO) see all in progress requests for their institution, while principal investigators (PI) see self-initiated, in progress requests or those routed to their attention by an SO. To narrow results, the SO can type text in the Filter Table field to see matching results, or use the Search for Requests button to enter search criteria; see Figure 1.
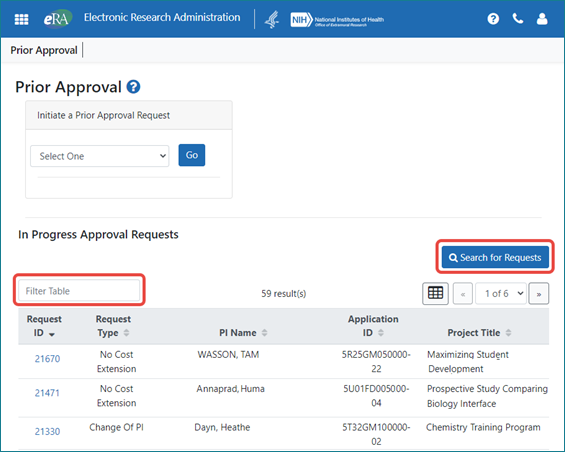
Figure 1: The SO view, showing all in progress requests for the institution.
PIs Use Quick Toggles to Filter
PIs, who typically have few in progress requests, can narrow the results shown by using three quick toggles; see Figure 2.
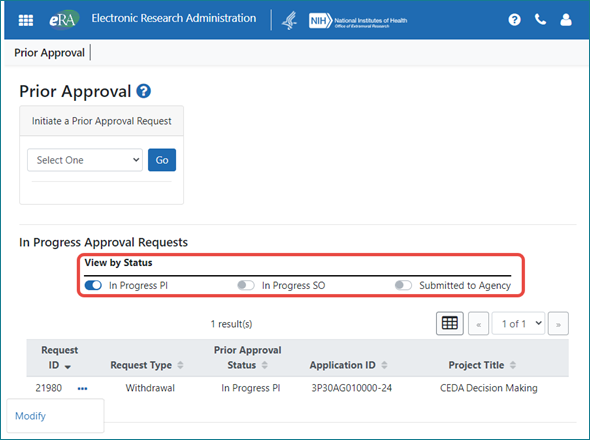
Figure 2: The PI view, showing in progress requests with quick toggles for filtering.
New Three-Dot Ellipsis Icon for Performing Actions
When actions are available on data, look for the new three-dot ellipsis icon, which shows available actions; see Figure 3. The three-dot ellipsis menu is dynamic and shows only those actions that are currently available.
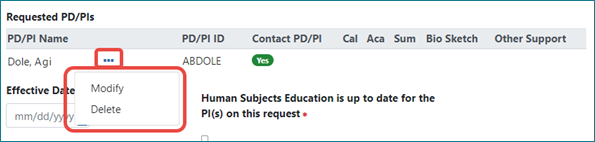
Figure 3: The new three-dot ellipsis icon showing a menu of available actions.
Please look for updated content following the March 7 release in the Prior Approval topic in the eRA Commons online help.
January 14, 2022
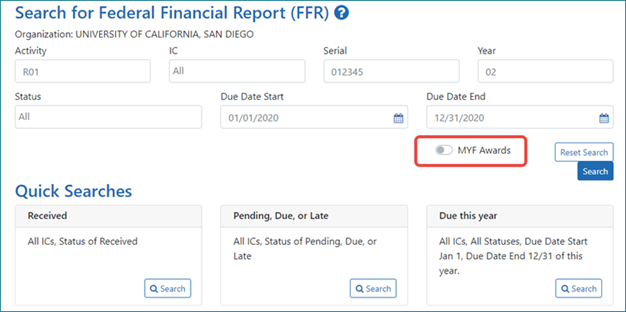
eRA Enhancements: New Look Coming January 20 to Federal Financial Report Module in eRA Commons
- Apps menu icon — The apps menu, when clicked, shows a dropdown menu of other eRA modules available to the user.
- Buttons — If multiple buttons exist in a row, the primary button will be prominent and the secondary ones will either be outlined or have no border, to help highlight the options that are more likely to be used.
- ·Header and Footer — An elegant header and footer take up less space, leaving more real estate for the functionality of the module.
- ·Action items — A three dot ellipsis icon, when clicked, displays a dropdown menu of action items. This icon replaces the action column. See Figure 1.
2018
Oct. 18, 2018
- Personal Profile on the Go — A mobile version of the Personal Profile screen will be available after the release, giving eRA Commons users access on the go. In addition, security has been enhanced for the entire Personal Profile.
- No Zero for Person Months — A zero can no longer be entered for person months as level of effort in Section D.1 -Participants of the Research Performance Progress Report (RPPR). The zero reflected a time when only whole numbers were allowed, and users were asked to enter a zero if a person month effort for a participant was 0.4 or less. Since decimals are now allowed, the zero is no longer needed.
- Security Enhanced — Security for eRA Commons has been enhanced to keep up with the latest technological advancements.
2017
September 2017
Interim Requests for Additional Materials (IRAMs) may now be processed in Commons
June 2017
8th - Carryover requests are now available in the Prior Approval module
February 2017
Prior Approval
- 21st - No Cost Extensions now available in the Prior Approval module
- 23rd - Change of PD/PI is now available in the Prior Approval module
2017
January 2017
- Final RPPR Section D.1 Added
- As part of the transition to the use of the Final Research Performance Progress Report (FRPPR), Section D.1 of Participants is now included.
- Section D.1 asks “What individuals have worked on the project?” This information will be the list of people who have worked on the project since the previous progress report. It does not include all the individuals during the lifetime of the award.
- As part of the transition to the use of the Final Research Performance Progress Report (FRPPR), Section D.1 of Participants is now included.
- New Design of the Status Information Screen for PIs and SOs
The Status Information screen will have a new look and feel. The screen is an important source of information for Principal Investigators (PIs) and Signing Officials (SOs) for such things as scores, summary statements, NIH contacts, reference letter status, etc.
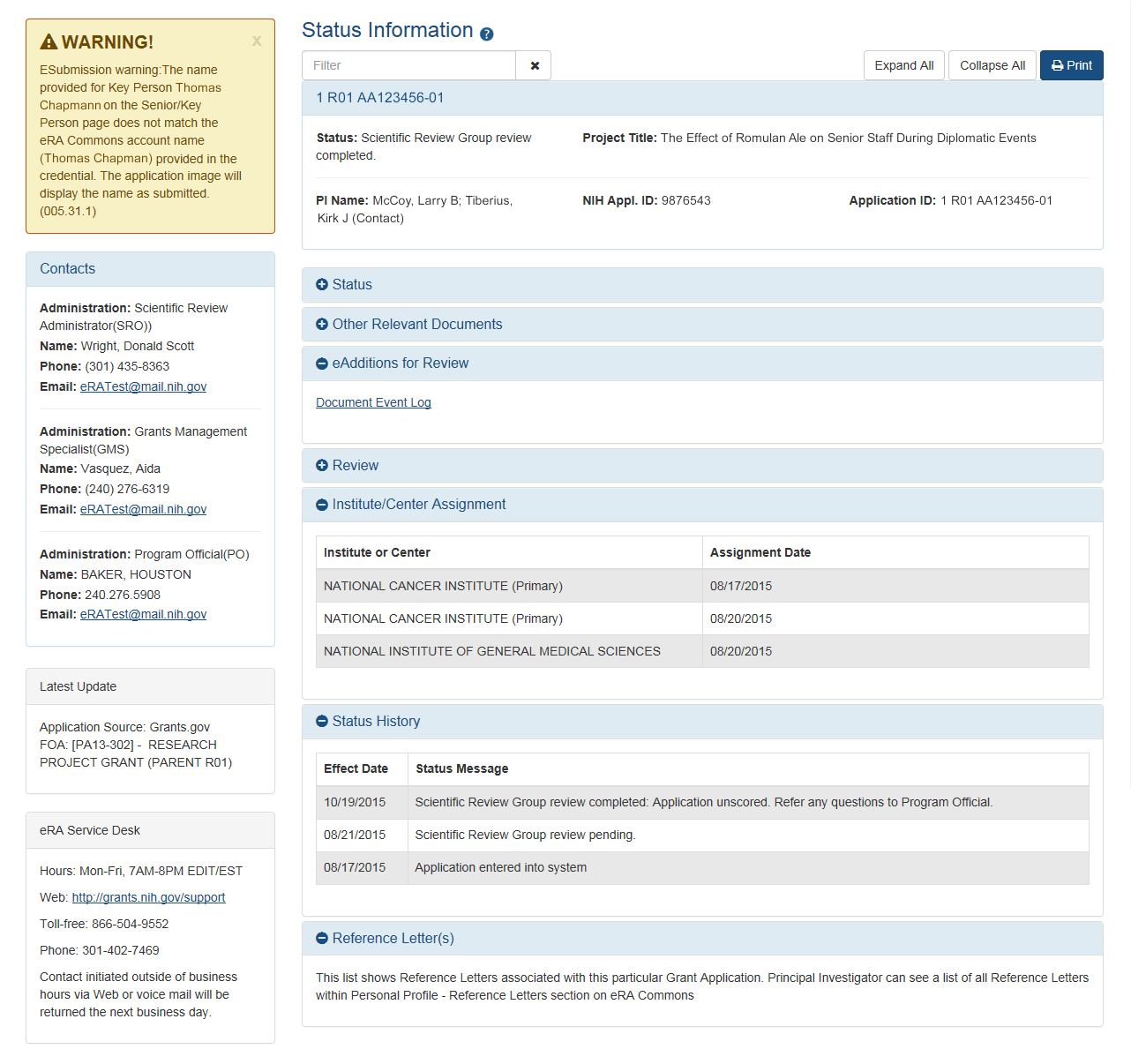
New Status Information screen with some sections expanded, other collapsed.
- The new design is similar to other updated screens, such as the Personal Profile and the Institution Profile
- Categories of information are organized into collapsible/expandable sections
- Expand All (default view) and Collapse All buttons show and hide the information on the screen
- New Text Filter
- A new field will allow you to enter text and display the results on those sections with matches
- Matching text will be highlighted, allowing you to quickly locate the information
- Important information is displayed along the left side:
- Warnings
- Contact information
- Scientific Review Officer (SRO)
- Grants Management Specialist (GMS)
- Program Official (PO)
- Latest Updates
- eRA Service Desk contact information
- New Print Capabilities
- New formatting capabilities allow you to print all the information with the click of a single button
- Organized by category
- Includes warnings
- Includes NIH contacts
- Final RPPR To Be Used Effective Jan. 1, 2017
- The Final Research Performance Progress Report (F-RPPR) will replace the Final Progress Report (FPR) for grants closeout, effective January 1, 2017. The F-RPPR will be available for use in eRA Commons on January 1, 2017.
- NOTE: For small businesses, the new F-RPPR will be in effect at least 2 months later, due to the unique final reporting requirements that they face under the SBIR/STTR policy directive.
- What This Means for You
- If you have a final progress report due, and you wish to use the old FPR format of an uploaded document, you must submit the FPR before January 1, 2017. NIH will no longer accept any of the old format FPRs on or after January 1, 2017.
- The Format
- The format of the Final RPPR is very similar to that of the annual RPPR. The notable differences being the F-RPPR only uses section D.1 for Participants, and does not use sections F (Changes), and H (Budget). The F-RPPR does have a new section: Section I (Outcomes).
- Project Outcomes (Section I) will be made publicly available, allowing recipients the opportunity to provide the general public with a concise summary of the public significance of the research.
- Deadline Remains Unchanged
- The deadlines for submitting a Final RPPR remain the same – no later than 120 days from the project end date.
- The Effect on Delegation to Submit RPPR
- NIH will maintain the business rule that allows the Signing Official (SO) to delegate the submission of the Final RPPR or Interim-RPPR to a Program Director/Principal Investigator (PD/PI).
- What This Means for Inclusion Data Reports in IMS
- The ‘last budget period’ Inclusion Data Records (IDRs) will be replaced with ‘final’ IDRs. If IDRs already exist, final IDRs will now be automatically created by the system for the last support year of each competitive segment.
- Users will be able to edit existing final IDRs. If additional IDRs need to be created, users can create them using the existing method.
- Once the grant is closed, this ability to create or edit IDRs will no longer be available. The Manage Inclusion Data Records (IDRs) screen in IMS will display a ‘Grant Closed’ message. At that point, users will be able to only view final IDRs.
- Accessing IDRs for the Final RPPR is the same process as for annual RPPRs. When the Final RPPR is initiated, users will go to Section G.4.b and click on the Inclusion link. This action takes you to the Inclusion Manage Inclusion Data Records (IDRs)screen.
2016
October 2016
- SAMHSA will now use Commons to initiate, track, and manage the progress of non-research amendment applications.
- This functionality will now be found in the "Manage Post Award Amendments" module under the "Non-Research" tab for eligible users.
- When a user initiates a non-research amendment in Commons, the system will open up the application, with the appropriate forms, in ASSIST and the completion and submission of the application will happen there.
- Subsequent to submission, the user will continue to; track the application process; submit, view, and edit "Requests for Additional Materials" (RAM); and view amendments in Commons.
- New Non-Research Tab Has Been Added to the Top Navigation
- As part of the expansion of eRA services to other federal agencies, the Non-Research tab has been added for recipients of SAMHSA (Substance Abuse and Mental Health Services Administration) non-research grants.
- The new tab is located after eRA Partners.
- Only those of you who also receive SAMHSA non-research grants will need to access this tab to manage post award amendments.
- SAM Registration Expiration Data Displayed
- The expiration date for institutions’ System for Award Management (SAM) registration will now be displayed on the Institutional Profile,
- The SAM registration needs to be renewed yearly and is required to successfully submit a federal grant application to Grants.gov.
- Redesigned Section in RPPR to Help Grantees Categorize Products Developed Under a Grant
- Grantees filling out a Research Performance Progress Report in eRA Commons are expected to list significant products developed with their grant in Section C of the RPPR, namely websites, technology and other products.
- The product sections of the RPPR have been redesigned recently to help grantees better categorize the products arising from their grant. A new scrollable menu lists 14 product categories under which to list the product(s): Audio or video; data or databases; research material; educational aids or curricula; evaluation instruments; instruments or equipment; models; physical collections; protocols; software; survey instruments; interventions (e.g. clinical or educational); new business creation and other.
- After entering a description for one or more reportable products in a text box, the grantee can then select appropriate categories for the product using a scrollable menu. Grantees are not limited to a single category per product; please select as many categories as appropriate for the product being reported.
- In addition, grantees can turn to a new resource ‘Guide to Categorizing Products in RPPR’s Section C’ to find definitions, examples and distinctions to help them decide which categories are appropriate for their product(s). For instance, videos developed to elicit behavioral change, such as counseling or motivational videos, or to instruct patients, may also meet the definition of clinical intervention.
July 2016
eRA Enhancements: eRA Commons’ Upcoming Release and Scheduled Downtime for Thursday, July 21
Wednesday, July 20, 2016
- New ASSIST Tab for Signing Officials (SO) and Principal Investigators (PI)
- As part of the top navigation menu, an ASSIST tab will be available to SOs and PIs
- The new tab will be located between the Status tab and the Prior Approval tab
- Selecting the tab will launch ASSIST in a new window and automatically authenticate the user, providing immediate access to ASSIST
- New RPPR Feature for Publications
- When selecting/deselecting publications for RPPR submissions, users will see a gold lock icon next to those publications which cannot be removed from the RPPR
- To remove the grant affiliation from the publication with a gold lock, users will need to contact the NIHMS help desk through their web form, which is accessible at http://www.nihms.nih.gov/.
February 2016
eRA Enhancements: Highlights of eRA Commons' xTRACT Release Scheduled for Thursday, February 4
Wednesday, February 3, 2016
eRA Commons' Extramural Trainee Reporting And Career Tracking (xTRACT) system will undergo a maintenance release this Thursday, February 4, 2016. These are just some ofthe features being rolled out for this release; check out the release notes, following the release, for more information on features and fixes.
NOTE: The maintenance process will not affect other aspects of the production environment for eRA Commons.
New Features
- Subsequent Grants Updated for Trainee or Student When Training End Date is Entered
- When a training end date is entered in the End Date (when Trainee Left Program) field on the Participating Trainee Detail screen for a participating trainee or student, the subsequent grants for that trainee or student are prepopulated from available NIH sources provided that no subsequent grants were previously entered for that trainee or student for that particular Research Training Dataset (RTD).
- Added Feature for Uploading Non-NIH Funding Sources is now Available
- A new feature in xTRACT for uploading non-NIH funding sources is accessed by clicking the Institution Data tab and clicking on the Upload Funding Sourceshyperlink.
Fixes
- Commons User ID now Displaying in Participating Trainees List
- Previously the Commons User ID was not always displaying in the Participating Trainees list for a Research Performance Progress Report's (RPPR) RTD.
- Non-NIH Sources of Support now Displaying Correctly in Preview PDF
- Previously the non-NIH sources of support were being displayed incorrectly in the PDF when the Preview PDF hyperlink was clicked in xTRACT. These sources of support applied to any non-NIH sources of support under the Summary of Support During Training in the following tables:
- Table 8A – Program Outcomes: Predoctoral
- Table 8C – Program Outcomes: Postdoctoral
The correct abbreviations listed below now display:
NSUnivNon-USOther FedFdnOther
- Previously the non-NIH sources of support were being displayed incorrectly in the PDF when the Preview PDF hyperlink was clicked in xTRACT. These sources of support applied to any non-NIH sources of support under the Summary of Support During Training in the following tables:
- Training Grant Search now Displaying Type 5 Grants Correctly
- Previously when a Type 2 (competing renewal) grant application had been submitted and its status was Pending Review, the previously awarded Type 5 in the grant family was not appearing in the search results on the Search for Training Grants screen. Consequently, if a user wished to start preparing for a resubmission of the competing renewal, there was no way to do so in xTRACT.
- The preceding awarded Type 5 grant is now returned in the search results on the Search for Grants screen.
- OTH Degree Text is now Displaying in the Training Table PD
- If a faculty member or trainee/student received an OTH (Other) type of degree, it is required to enter what type of degree was earned in the Other Degree Text field on various screens in xTRACT. This Other Degree Text was not being displayed where appropriate in the Training table PDF.
- The Other Degree Text applies to any degrees listed in the following tables:
- Table 8A – Program Outcomes: Predoctoral
- Sections: Terminal Degree(s) received and Year(s)
- Table 8C – Program Outcomes: Postdoctoral
- Sections: Doctoral Degree(s) and Year(s) or Degree(s) resulting from Postdoctoral training and Year(s)
- Table 8A – Program Outcomes: Predoctoral
- Doctoral Degrees now Appearing in Training Table 8C
- Previously doctoral degrees were not appearing in the column labeled Doctoral Degree(s) and Year(s) in Table 8C whenever the doctoral degree earned date and the postdoctoral trainee's entry program date were in the same month and year.
- When Performing Training Grant Searches Assistants can now See all of their PIs
- When performing training grant searches Assistants were not always seeing all of their Principal Investigators (PIs) as expected in their Delegator List in xTRACT.
January 2016
New Features
Updates in eRA Commons
NIH has added several new features to eRA Commons:
- Principal Investigator (PI) Screens Redesigned for Status, JIT, and Closeout
- The eRA Commons’ Status screen for PIs has been redesigned with a refined interface, a responsive design and grouping of information that is user friendly. Searches on the Recent/Pending eSubmissions and List of Applications/Grants screens will now provide a total count of applications/grants at the top of the screen. The results will be grouped by Application ID/Grant number and will be expandable and collapsible as needed.
- Additionally, eRA has updated the look of both the Just in Time (JIT) and Closeout screens for PIs.
- Both have been reconfigured to improve readability and flow.
- Both now support “Drag & Drop” functionality to upload files while dynamically validating file size and format.
NOTE: Signing Officials will see this same functionality once the redesign of the SO Status Screen is completed later this year.
Also a condensed version of the Status screen for PIs is now available for use on mobile devices through a special URL — https://m.era.nih.gov/cmb. See eRA News for more information on this topic.
- Electronic Signature of New Organization Registration in eRA Commons
- The registration process for eRA Commons will be made less burdensome: Signing Officials will now be able to sign electronically instead of signing a hard copy and faxing the registration form to eRA.
- New Functionality for Delegates
- Delegates will benefit from new functionality within eRA Commons. If Status is delegated to an person from multiple PIs, the selection of the person for whom they are acting will remain throughout their connection to Commons unless they specifically change the Status setting.
2015
October 2015
New Features
Update in eSubmission
- Diversity Administrative Supplements Now Require Credentials
- When submitting Diversity Admin Supplements (Type 3) applications, all Senior/Key Persons are required to have valid eRA Commons credentials.
- Failing to provide valid credentials for Senior/Key person will result in the following error message: The eRA Commons Username provided for is not a recognized eRA Commons Account.
- This error will result in the application being rejected for further consideration.
Updates in eRA Commons
NIH has added several new features to eRA Commons:
- eRA Help Desk Now eRA Service Desk
- The eRA Commons Help Desk has been rebranded as the eRA Service Desk.
- Commons users will see this change on screens, email notifications, web pages, and other resources.
- The new name reflects the team's expansion to support ASSIST and other eRA services.
- New Final Invention Statement Notification(s) to Institutions
Institutions will now receive email notifications that Final Invention Statements (FIS) are ready for acceptance by a Signing Official (SO) and submission to agency.
- eRA Commons will send an initial email after a PI or SO has uploaded an FIS and it is in a status of Awaiting SO Verification. This email will be sent to the Closeout Correspondence email address (as captured in the IPF), as well as the Announcement and Notifications email address (as captured in the IPF).
- If after 10 days the SO has not submitted the FIS to agency, Commons will send a follow-up reminder email to the Closeout Correspondence email address.
The following parties are included on the notifications:
- PI who created the FIS
- NIH Closeout Center
- Closeout Correspondence Email address
- Announcement and Notifications email
- Signing Official (only if an SO has uploaded information for the FIS)
- Security Precautions added to DOB and SSN Fields
- eRA has added extra security to the Personal Profile for the Date of Birth and Social Security Number (SSN) fields.
- These two fields are now only editable if blank or filled with all zeroes.
- Once populated, the fields will become read-only.
NOTE: Should you need to make changes to either of these fields on your Personal Profile, contact the eRA Service Desk for assistance.
- Confirmation Checkbox Added to the Extension Screen
- Users submitting a No-Cost Extension (NCE) will notice a new checkbox that verifies that the terms of the NCE have been read and are understood as follows:
By notifying NIH of this one-time extension of the period of performance, you certify that the extension is not: 1) being exercised merely for the purpose of using unobligated balance; 2) prohibited by the terms and conditions of the Federal award; 3) requesting additional Federal funds. Further, it does not involve any change in the approved objectives or scope of the project.
- Selecting the checkbox enables the Confirm button for submitting the request. You must select the checkbox to confirm.
- Introducing xTRACT
- The xTRACT module will be available via the eRA Commons (See Overview).
- It will allow applicants, grantees, and assistants to create research training tables for progress reports and institutional training grant applications.
- Initially, xTRACT will support the following training grant award mechanisms: T32, TL1, T90/R90, and T15 for both progress reports and grant applications.
NOTE: xTRACT can be used on a pilot basis for creating data tables for those mechanisms for Research Performance Progress Reports due Dec. 1, 2015 or later and applications submitted for the May 25, 2016 due date and after (See Guide Notice NOT-OD-16-007).
For details, please see the latest eRA Commons Release Notes.
July 2015
New Features
- Expansion of the Federated Login Functionality
- Federated Login is the ability for users to use their institutional user ID and password to access eRA Commons. This functionality has now been expanded to include users with the ASST role in eRA Commons.
- This option is for organizations currently registered with InCommon and who work with NIH’s Center for Information Technology (CIT) to establish the relationship between your institution and eRA Commons.
- For more information on the Institution/Organization Log In functionality, review the October 17 Commons Release Notes, or the Commons Online Help System.
- New Closeout Requirements for Federal Financial Report (FFR)
- The eRA Commons link for the final Federal Financial Report will reflect that the deadline for submitting the report is 120 days after the Budget Period End Date, for projects that ended on or after Oct. 1, 2014
- This is an increase from the previous 90 days for all final reports as part of the Closeout process (see video tutorial) and Uniform Guidance.
- xTrain Notifications for Fellowships Now Available on the Status Information Screen
- Reminders for fellowship terminations are sent 30 days prior to the project end date, 1 day after the project end date, and 30 days after the project end date.
- These fellowship notifications will be viewable by the grantee on the Status Information screen.
- NOTE: These notifications for National Cancer Institute (NCI) fellowships will not be available in Grant Folder until September 2015.
For details, please see the latest eRA Commons Release Notes.
May 2015
New Features
Termination Reminder Notifications are now Being Activated for Training Grants with T Activity Codes
- If a trainee is not reappointed, Termination Reminder notifications are now being sent to the appropriate Principal Investigator (PI) for training grants (T activity codes) as follows:
- 30 days before the appointment end date
- 1 day after the appointment end date
- 30 days after the appointment ended
Fix
Termination Notifications are now being Sent Correctly per Grant Conditions and Time Frame
- Previously Termination Reminder notifications were being set incorrectly as follows:
- Termination Reminder notifications were sent when there was a future reappointment in an Accepted status.
- Termination Reminder notifications were sent thirty days after the appointment end date stating that there was thirty days left before the appointment end date. The notification should have stated that thirty days had passed since the appointment end date.
- Multiple Termination Reminder notifications were sent for the same grant applications.
April 2015
- Unilateral Closeout
- Modifications to Status–Closeout Search Screen and Results for Unilateral Closeout
- The U.S. Department of Health and Human Services (HHS) has issued a directive to Agencies on new policies for closeout of grant awards. NIH has revised its policies and procedures to align with the OER Policy Announcement 2014 regarding the guidance on implementation of HHS GPAM Chapter 1101 (Closeout), including Unilateral Closeout.
- As a result of this directive, the Status Closeout screens have been modified.
- Two new statuses have been added to the Closeout Status search parameter on the Closeout Status search screen to limit search results to these grants.
- In Unilateral Closeout
- Unilaterally Closed
- The Status Result – Closeout Search screen will include the following links for grants unilaterally closed or in the process of being unilaterally closed. The links open the Closeout Status screen.
- In Unilateral Closeout
- Unilaterally Closed
- Two new statuses have been added to the Closeout Status search parameter on the Closeout Status search screen to limit search results to these grants.
- Modifications to Status–Closeout Search Screen and Results for Unilateral Closeout
- Financial Conflict of Interests (FCOI) Updates
- Administrative Supplements Removed from Search Results
- In FCOI Module, Grantees can no longer initiate Financial Conflict of Interest reports for Administrative Supplements.
- Modified for Institutions with an Inactive FCOI Official
- Commons will now verify that an organization’s FCOI official on record continues to maintain an active Commons FCOI account within that organization before emails are sent to that person. If the official is no longer with the organization, or if the account’s roles no longer include FCOI, the following will occur:
- The Signing Official (SO) will be copied on the communication
- An active FCOI official will be copied on the communication. Commons will select the first FCOI official, alphabetically in the organization
- Commons will now verify that an organization’s FCOI official on record continues to maintain an active Commons FCOI account within that organization before emails are sent to that person. If the official is no longer with the organization, or if the account’s roles no longer include FCOI, the following will occur:
- Administrative Supplements Removed from Search Results
All emails going out to Grantees will include the following note:
You may be receiving this email communication since the FCOI official originally responsible for this FCOI report appears to be no longer affiliated with your organization or does not currently have the FCOI role. Therefore, please forward this communication to the appropriate FCOI official within your institution that is currently responsible for reporting identified FCOIs to the Agency.
- Federated Login Update
- Principal Investigators (PIs) logged in as Federated users will see the same features and modules in Commons as they do when logged in with Commons credentials.
- Institution Profile (IPF) Update
- Institution Profile Modified for Animal Welfare Assurance Number Management
- The Office of Lab Animal Welfare (OLAW) and eRA are collaborating to improve the capture and validation of Animal Welfare Assurance Numbers. The Institution Profile in Commons has been modified to support this collaboration.
- Institutions are no longer able to add or edit the Animal Welfare Assurance Number field. This field, located within the About the Institution panel of the Institution Basic Information screen, will display all assurance numbers for the institution as passed to Commons from OLAW system.
- Institution Profile Modified for Animal Welfare Assurance Number Management
- New Just-in-Time (JIT) Features
- JIT Feature Modified for Genome Data Sharing Certifications
- The Just in Time (JIT) screen has been modified to allow the upload of an Institutional Certification for Genome Data Sharing.
- JIT Feature Modified for Genome Data Sharing Certifications
- New xTrain Features
- PIs and PI Delegates Can Recall TNs Regardless of Last Reviewer
- PIs can now recall Termination Notices (TN) at any time, regardless of the current reviewer, as long as the TN has not been submitted to the Agency.
- xTrain Communications Display Grant Extension
- Grant numbers within all xTrain communications now include the grant extension number (e.g., 01A1) if one exists.
- PIs and PI Delegates Can Recall TNs Regardless of Last Reviewer
- No Cost Extension (NCE) Update
- In the January system-wide release, a free text field was added to the No Cost Extension form as a place for grantees to provide justification for the extension as required under HHS Uniform Guidance.
- For this release, the field has been removed to provide time for the development of a more elegant and user friendly option for meeting this requirement.
- In the January system-wide release, a free text field was added to the No Cost Extension form as a place for grantees to provide justification for the extension as required under HHS Uniform Guidance.
For details, please see the latest eRA Commons Release Notes
January 2015
- Single-project Applications
- Added a direct cost limit calculation to the application image, so users can determine if their application falls within any direct cost limits of a Funding Opportunity Announcement.
- Extension Justification
- NIH will be requiring an explanation for grantee-initiated extensions be provided in a new 2000 character limited text box, when requesting a No Cost Extension. The text box will make it easier and more convenient to provide justification for the extension.
- New Security Confirmations
- NIH takes security very seriously. To that end, the following new features are designed to ensure your personal information on the Personal Profile form remains protected:
- A change to your Social Security Number, Date of Birth, or Contact Email Address will trigger a confirmation message to the original email address, alerting you to the change of information.
- When changing either the Social Security Number or Date of Birth, the system will display a pop-up window confirming that you want to make the change.
- NIH takes security very seriously. To that end, the following new features are designed to ensure your personal information on the Personal Profile form remains protected:
- New Project Title Limitations
- Project titles can now support scientific characters such as Greek letters and/or mathematical symbols. However, while eRA Commons will support the use of these characters, it will not be until February 2015 that Grants.gov will complete its systems upgrade to support these characters. Please look for an upcoming Guide Notice for more information.
- New Closeout Functionality
- On the Institution Profile form, under Institution Contact Information, there will now be a new required field for Closeout Correspondence Email.
- For existing institutions, the Notice of Award Email address will be automatically copied to the Closeout Correspondence Email field until it can be updated.
- For closeout related roles (AO, PI and SO), emails sent to these users will also be CC’d to the Closeout Correspondence Email.
- Federal Financial Reports will require comments when a revision is submitted.
- Final Progress Report (FPR) submission can be delegated and for existing users with the RPPR submission delegation, the FPR submission delegation will be added automatically.
- New RPPR Functionality
- There is a new Recall button for Agency PRAM requests. Like other recall functionality, the last reviewer can recall the Agency PRAM (formerly IC PRAM) if the PRAM status is not “Submitted to Agency.”
- For complex (multi-project) applications, the new PHS Additional Indirect Costs form will be available for projects with an Overall Component and at least one subcomponent.
- New xTrain Features
- A new status for fellowships has been added: Proxy Termination. When a Proxy Termination is accepted by agency for a non-fellowship appointment, the status will now be displayed as Proxy Termination.
- The Business Official (BO) will now have the ability to modify the stipend amount. However, this is only available when the appointment period has been modified.
- Reminders sent to Principal Investigators (PIs) for trainee terminations will be sent 30 days prior, one day after and 30 days after the termination date.
- Automatic termination of trainees for accepted appointments prior to January 1, 2011. In an effort to clean up legacy data, the system will automatically terminate appointments that were accepted by an institution prior to January 1, 2011. The “Reset to Prior State” option will not be available for these appointments. If you need assistance for an appointment that gets terminated as a result of this process, please contact the Help Desk.
- Financial Conflict of Interests (FCOI) Updates
- Enhancement
- User will now be able to leave comments/text for question # 5 (Retrospective Review Completed? N/Y) on the original FCOI report. Comments will be visible to Agency users.
- Critical Fixes
- Annual report link will now be correctly displayed on FCOI search results screen. Annual report due date calculation will now correctly be based on the Original FCOI submission dates and all the related information will be based on latest submitted FCOI record (Revision or past Annual reports)
- Based on policy, the system will not allow the user to submit an FCOI Revision if, on the original FCOI report, Question # 4 (Does this FCOI report include a failure to comply with the regulation? N/Y), is answered as ’Yes.’
- Enhancement
2014
October 2014
- Inclusion Management System (IMS)
- eRA will unveil a new system to report inclusion of women and minorities in clinical research and for NIH staff to monitor inclusion data. See Guide Notice NOT-OD-15-005 for more information. Grantees will provide inclusion enrollment data with competing submissions and will be able to access the new Inclusion Management System (IMS) through eRA Commons to create new inclusion records or view/edit/update existing inclusion data records. Inclusion data submitted through competing applications, eRA Commons, or the RPPR (Research Performance Progress Report) will automatically populate IMS.
- See the Inclusion Management System (IMS) Online Help, the IMS Guide or click on the question mark on the IMS screens for more information, following the release. Also check out the inclusion Policy Implementation Page.
- New Closeout Procedures & Policies
- HHS guidance on closeout of grant awards has prompted changes to improve communications, efficiency and policy compliance throughout the closeout process.
§ New Features for Grantees- The ability to identify grants that will be closing soon. A new 'quick query' on the eRA Commons home screen will allow grantees to view grants approaching the project end date. This feature will not require Commons login.
- The ability for grantees to submit any additional information sought by NIH through a feature called "Final Report Additional Material" (FRAM). Mirroring the functionality of Progress Report Additional Materials (PRAM) requests, grantees can upload FRAM data multiple times for multiple FRAM requests, but one upload per request.
- Expanded notification to grantees throughout the closeout process. Previously, one notice was sent to the grantee after the project end date, reminding them of the reporting requirement, followed by a second notice identifying any overdue final reports. These messages have been revised to emphasize the criticality of completing the closeout process; and a third notice will be added to address any remaining overdue or unacceptable report submissions.
- HHS guidance on closeout of grant awards has prompted changes to improve communications, efficiency and policy compliance throughout the closeout process.
- Federated Login for eRA Commons
- eRA will pilot the use of organizational credentials for Commons login. The pilot will be limited to those organizations currently registered with the Center for Information Technology (CIT) and individuals with limited Commons roles. This pilot will allow an eRA Commons user to map his or her current eRA Commons account to the organizational login and then access eRA Commons with his or her organizational user name and password.
The pilot will consist of users with basic eRA Commons roles. The roles eligible for the pilot are:
PostDoc
Graduate StudentScientist
Project PersonnelTrainee
Under-Graduate
- eRA will pilot the use of organizational credentials for Commons login. The pilot will be limited to those organizations currently registered with the Center for Information Technology (CIT) and individuals with limited Commons roles. This pilot will allow an eRA Commons user to map his or her current eRA Commons account to the organizational login and then access eRA Commons with his or her organizational user name and password.
- Commons ID for Students
- Graduate and undergraduate students working on grants will need to have Commons IDs starting Oct. 1, to be included in the progress reports. The Research Performance Progress Report will receive an error and will not be accepted without this information (See NOT-OD-13-097)
- RPPR Required for All Non-SNAP Progress Reports
- Use of the Research Performance Progress Report (RPPR) is required for all type 5 non-SNAP progress reports submitted on or after October 17, 2014 (See NOT-OD-14-092)
- Individual Development Plans (IDPs) Needed in Annual Progress Reports
- Grantees will need to include a section on how individual development plans are being used to "identify and promote the career goals of graduate students and postdoctoral researchers associated with the award." (See NOT-OD-14-113)
July 2014
Updates in eSubmission
NIH has made several changes to enhance the usability of the Application Submission System & Interface for Submission Tracking (ASSIST) solution:
- Ability to Copy an Application in ASSIST
- The Copy Application feature has been added to ASSIST. This feature allows Signing Officials (SO) and Administrative Officials (AO) users to duplicate an existing application or components of an application (minus attachments) for submission under another Funding Opportunity Announcement (FOA).
- Ability to Delete Applications and Components in ASSIST
- Delete Application
- SOs will see the Delete Application button on the Actions panel while on the Application Information page. This button is used to permanently delete the entire application.
- Delete Component
- From the Summary tab of a component, SOs, AOs, and Principal Investigators (PIs) can select the Delete Component button to permanently delete a specific component. Deleting the component removes it from the application and updates the Application Status History to reflect the deletion.
- Delete Application
- Access to ASSIST Extended to All eRA Commons Roles
- Access to ASSIST has been modified to allow anyone with an eRA Commons role to log into the system using those same credentials. Previously, only certain eRA Commons roles were given access to ASSIST.
- Application Image Enhancements
- The PDF version of multi-project applications has been enhanced for greater usability. The Table of Contents now indents the forms and attachments of each component of a particular component type. This provides greater ease of navigation and improved readability of the Table of Contents.
- Unique File Names Required for Multiple Attachments on a Single Form
- A new file name restriction has been implemented for form attachments. When uploading multiple attachments on a single form, each file name must be unique. Duplicate file names may exist across multiple forms.
Updates in eRA Commons
NIH has added several new features to eRA Commons:
- Personal Profile Form (PPF)
- A new requirement when adding residency information to the Education component of your personal profile: you must also enter your degree information, if it does not currently exist. Failure to enter your degree information will result in additional changes not being saved and the following error message: Please enter degree information before entering residency.
- New Institution Profile Form (IPF)
- Using the same look, feel, and functionality of the Personal Profile Form released last year, the IPF is the central repository of information for all Commons registered applicant organizations. This newly re-designed module is sophisticated, clean, and intuitive and will vastly improve users' experience.
- Some features of the new Institution Profile include:
- Institution Basic Information displayed in components: Basic information about the institution is grouped into components by the type of data making specific data easy to find and maintain.
- Indication of missing information: Warning icons and messages highlight any required fields that are missing information.
- Ability to view or edit information: Two options – View and Edit – allow you to view a summary of information for each component of the profile or, if you are an SO, to access all information in a component to perform edits.
- Re-designed Assurances and Certifications: Easily updatable assurance and certification fields with clear checkboxes and explanation text fields.
- Support for Maintaining Multiple DUNS Numbers in IPF
- As part of the IPF redesign, institution officials are now able to maintain multiple DUNS numbers for their organization.
- The About the Institution component of the profile includes two fields: Primary DUNS and Secondary DUNS. The Primary DUNS number is read-only, populated by the original institution registration and not able to be edited. NOTE: If the currently displayed Primary DUNS number is not correct or needs to be updated, contact the eRA Help Desk at http://grants.nih.gov/support/index.html
- The Secondary DUNS number field(s) allows the SO to edit, add, and remove additional DUNS numbers for the institution as necessary. To change a Secondary DUNS number, just update the editable text fields.
- The About the Institution component of the profile includes two fields: Primary DUNS and Secondary DUNS. The Primary DUNS number is read-only, populated by the original institution registration and not able to be edited. NOTE: If the currently displayed Primary DUNS number is not correct or needs to be updated, contact the eRA Help Desk at http://grants.nih.gov/support/index.html
- As part of the IPF redesign, institution officials are now able to maintain multiple DUNS numbers for their organization.
- New RPPR Inclusion Forms
-
- For Research Performance Progress Reports (RPPR) requiring inclusion enrollment reporting, users can attach inclusion enrollment forms. The enrollment forms can be downloaded from the RPPR, completed by the user, and then added as a PDF attachment within Section G. Special Reporting Requirements (G.4.b specifically). This is a temporary process until the new Inclusion Management System is deployed in a future release.
- As we transition to the new inclusion report format, it is important to select the correct for to use. For awards with start dates BEFORE October 1, 2014, use the previous inclusion enrollment reporting format. For awards with start dates ON or AFTER October 1, 2014, use the updated inclusion enrollment format.
- See this Guide Notice for more information: http://grants.nih.gov/grants/guide/notice-files/NOT-OD-14-085.html
-
- Public Access PRAM Updated
-
- The Public Access Progress Report Additional Materials (PRAM) feature has been modified to require the addition of the My NCBI PDF report as proof of compliance with NIH Public Access Policy.
- Users select the Add Attachment button on the PRAM screen to upload the PDF.
- Attachments will be viewable within the PRAM PDF document.
- The Public Access Progress Report Additional Materials (PRAM) feature has been modified to require the addition of the My NCBI PDF report as proof of compliance with NIH Public Access Policy.
-
- Multi-Year Funded Award (MYF) RPPRs and Multi-Component Pre-population
- Users will find that many of the common fields of the MYF RPPR and Multi-Component will be pre-populated with data from the previously submitted report. This greatly reduces the burden on the grantee of completing the forms. See the release notes for a complete list of which fields get pre-populated.
April 2014
NIH is making several changes to the Financial Conflict of Interest (FCOI) module to improve institutional reporting and promote compliance:
- The FCOI Annual Report link in eRA Commons will appear closer to when it is due:
- For ongoing projects: The Annual Report link will appear 75 days prior to the next budget period start date, following the submission of an Original Report or Annual Report when there is a future noncompeting year pending within a current competitive segment.
- For eRA Commons No Cost Extensions: The Annual Report link will appear the day after the grantee takes action to extend the project via the Commons.
- For Notice of Award (NoA) extensions: An Annual Report link will not be available. Instead, until eRA implements a change in the FCOI module, the NIH will send the grantee a "Request for Additional Information" asking the grantee to provide a status of the FCOI and any changes to the management plan, if applicable, prior to the issuance of the revised NoA.
- FCOI Signing Officials (SOs) Will Receive an Email Reminder That Annual Report is Due:
- An email reminder will now be sent to the FCOI SO, when the Annual Report link becomes available in the FCOI module (as described above).
- FCOI Reports to be Submitted under the Current, Active Grant Year:
- When an FCOI SO enters a grant number for a grant year that is not the current, active grant year, a warning message will appear to allow the SO to select another grant year, if appropriate.
- Future Annual Reports will be submitted under the currently funded grant year (e.g., year 04) without regard to the grant year in which the Original Report was submitted (e.g., year 01). This is a change to the way the system currently functions.
- Revised FCOI Reports May Be Submitted up to 150 days After Submitting an Original Report:
- Revised FCOI reports are only needed following the completion of a Retrospective Review when the grantee needs to:
- provide new FCOI information that results in a change to a previously submitted FCOI report (e.g., an increase in value of a previously reported significant financial interest (SFI), discovery of a new SFI, or changes to the management of the FCOI, etc.) or
- provide a Mitigation Report if bias is found.
- Grantees should not submit a Revised FCOI report to notify NIH that either the Retrospective Review has been completed or that bias is not found.
- When the Original Report is submitted and the grantee indicates that the Retrospective Review is not completed, the system will allow the Institution to submit a Revised FCOI report up to 150 days after the Original Report's submission date.
- Revised FCOI reports are only needed following the completion of a Retrospective Review when the grantee needs to:
- New FCOI Roles in Commons Replace FCOI Delegations:
- Two new roles have been added to Commons for those who work with the Financial Conflict of Interest (FCOI) module. Only an organization's Signing Official (SO role) can assign these roles.
- FCOI_ASST: Assign the FCOI_ASST role to those users in the institution who will assist in working on the FCOI reporting process. These users can initiate, edit, view, search, and delete forms.
- FCOI_View: Assign the FCOI_View role to those users who need authority to search for and view FCOI information entered by the institution, but who will not need to perform any data entry or make changes to the information. These users will have read-only access to the FCOI report and data.
- Only an organization's Signing Official (SO role) can assign these roles.
- Two new roles have been added to Commons for those who work with the Financial Conflict of Interest (FCOI) module. Only an organization's Signing Official (SO role) can assign these roles.
Prior to this release, Commons users with the ASST role could be delegated the authority either to assist with FCOI tasks (FCOI Asst authority) or to view FCOI information (FCOI View authority). These new roles replace the former authorities, but provide the same levels of access to FCOI module. Starting with this release, SOs can assign a Commons user to one of the new roles and do not need to use the Delegation feature of Commons for FCOI purposes.
NOTE: With this release, eRA has assigned current ASST users delegated with either of the two FCOI authorities (FCOI Asst and FCOI View) to the corresponding new role as described above. For these existing users, the SOs do not need to assign the new roles.
When these corrections are needed, the FCOI SO should contact the Grants Management Specialist/Officer identified on the latest Notice of Award to ask NIH to rescind the FCOI report.
- Errors to the following data can be corrected by NIH sending the Institution a "Request for Additional Information," which allows the grantee to make the correction and submit the corrected FCOI report:
- FCOI data (entity name and significant financial interest data)
- Responses to the compliance questions (i.e., questions numbered 4, 5 or 6).
When these corrections are needed, the FCOI SO should write to the NIH Grants Management Specialist/Officer identified on the Notice of Award to explain the nature of the error and the need to make a correction.
- FCOI Corrections - A Change in Business Process when Grantees Identify Errors in Submitted Data:
- Corrections to some FCOI report data can be made without NIH taking action to rescind the Original report and requiring the grantee to submit a new report. Depending on the error, the options are:
- Errors in the following data can be corrected only by NIH rescinding the Original Report and requiring the grantee to submit a new report:
- Grant Number,
- Investigator Name with the conflict, and
- Subrecipient information
- Other FCOI Changes:
- Only one revision will be allowed to the original FCOI report
- Revisions to annual reports will no longer be accepted
- If NIH rescinds any FCOI record, the entire FCOI record (original, annual and revised reports) will be rescinded
For more information on these changes in FCOI, please see Guide Notice NOT-OD-14-081, the FCOI Online Help (accessed by clicking on the question marks on the screens of the FCOI module) and the FCOI User Guide.
- RPPR Open for all Type 5 Non-SNAP Progress Reports
Beginning on April 25, NIH will open up the use of the Research Performance Progress Report (RPPR) in eRA Commons for the electronic submission of annual progress reports for all Type 5 non-SNAP progress reports. For more information, please see the Submit Progress Report page, the Guide Notice NOT-OD-14-079, and the updated NIH Research Performance Progress Report (RPPR) Instruction Guide. - Online Help Now Available from Within eRA Commons
- Online Help for Account Management, used by authorized eRA Commons users to create and manage accounts, can now be accessed from specific Account Management forms within eRA Commons. The question marks for help will take you to the specific information for that form or topic.
- Federal Financial Report (FFR) also has new online help topics. FFR topics can be accessed from the Contents panel of the online help or by selecting one of the help ('?') icons found on most screens.
Updates in eSubmission
- Reorder Components in ASSIST
ASSIST now provides the option to set the order of different components within a component type. Previous to this release, components had to be created in the order in which you wanted them to appear in the final application image. You didn't have a lot of options if you entered 6 projects and then decided that you wanted the fourth project to appear first. Starting with this release, the order of the components (e.g., Project-001, Project-002, etc.) can be controlled and modified before final submission.
Refer to the online help for ASSIST for more information regarding this and all ASSIST features.
NOTE: The ability to submit help tickets online will not be available during the downtime listed above. However, customers can always email the eRA Help Desk at that time; commons@od.nih.gov (for eRA Commons support).
January 2014
The following are highlights of eRA Commons' upcoming January 30-31 system-wide software release. These are just some of the many features being rolled out for this release; check out the Release Notes for more information on features and fixes.
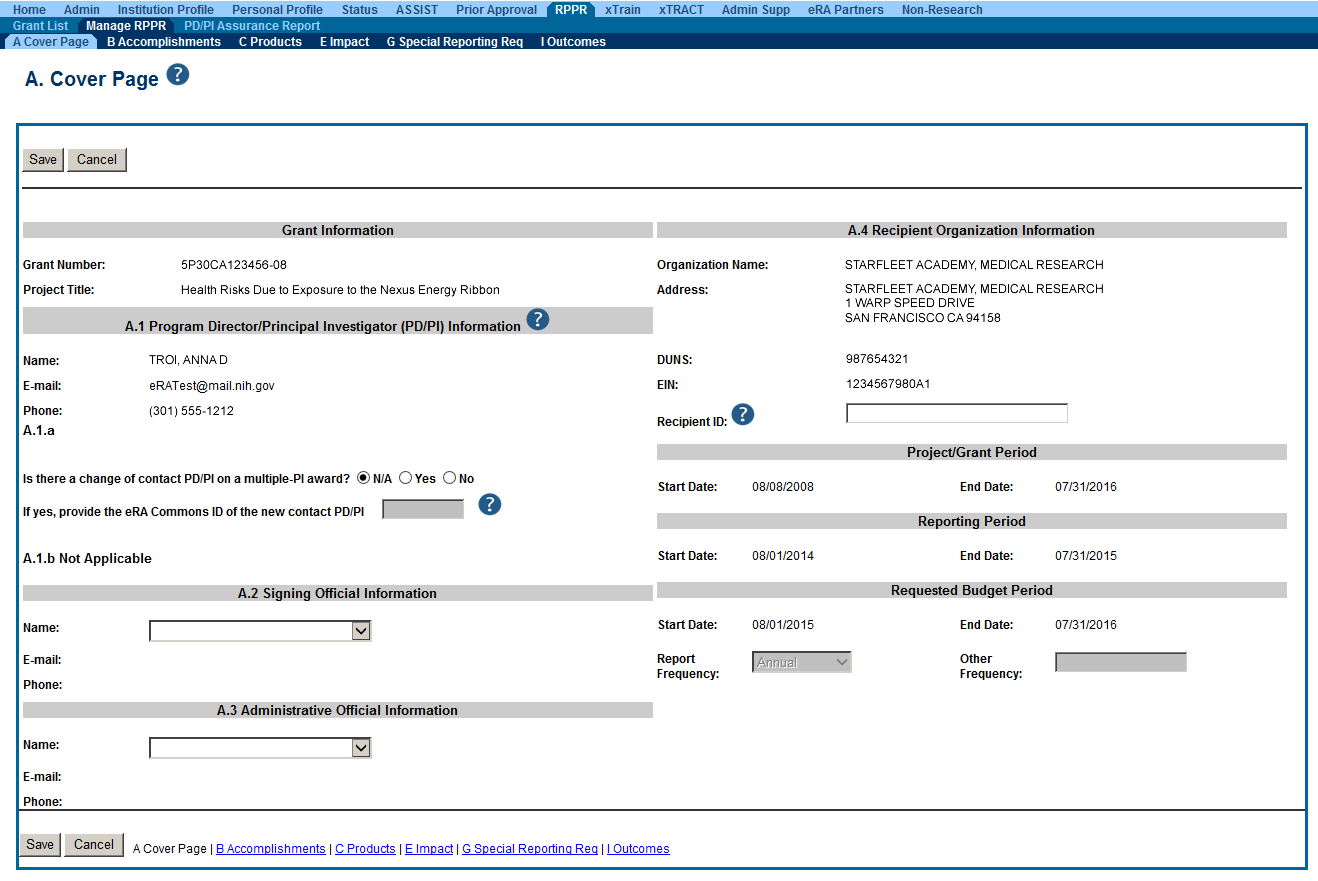
A Reminder About RPPR and Multi-year Funded Grants
- Changes in Research Performance Progress Report (RPPR):
With this release, NIH multi-year funded (MYF) awards must be submitted using the RPPR module in eRA Commons. This change goes into effect January 31, 2014. Grantees are able to initiate multi-year funded award progress reports via the Status screen in eRA Commons. For more information, please see the Submit Progress Report page, the Guide Notice NOT-OD-14-026, and coming soon will be the updated NIH Research Performance Progress Report (RPPR) Instruction Guide that will be available on or about the time of the release. - Project Title Field Expanded
With this release, eRA systems and databases will be able to accept grant application titles up to 200 characters. The project titles will no longer be truncated to the previous limit of 81 characters. Not all eRA screens and reports will reflect the expanded project title with this initial implementation. Over time these will be updated.
NOTE: When submitting a Revision application, applicants must use the exact project title displayed in eRA Commons for the awarded application. If the project title of the awarded grant was previously truncated to 81 characters, then only those 81 characters can be used for the Revision application. - Improved Continuous Submission Eligibility Information
Principal Investigators (PIs) and Signing Officials (SOs) will be able to view real time eligibility for continuous submission within eRA Commons. - PIs Access to Continuous Submission Eligibility Information
Principal Investigators will go to the Personal Profile tab of eRA Commons. The Reviewer section of the Personal Profile displays the Continuous Submission Eligibility information. PIs can review their eligibility based on the current appointed membership and/or recent substantial service. - SOs Access to Continuous Submission Eligibility Information
Signing Officials will go the Admin>Accounts>Advanced Search within eRA Commons. On the Account List form, they can search for a PI by Commons ID and/or name. The Account Search Results includes a column for CS Eligibility Details. The column will display Yes or No. These will be hyperlinked to a summary of the PI's Continuous Submission Eligibility status. - Administrative Supplements Online Help Now Available from Within eRA Commons
Online Help for Administrative Supplements can now be accessed from specific Administrative Supplement screens within eRA Commons. The question marks for help will take you to the specific information for that screen or topic. Previously, Administrative Supplements help was only available by launching the Commons Online Help from another screen and searching for Administrative Supplement topics.
2013
October 2013
This is just a sampling of the features being rolled out for this release and will be available Thursday, November 7, 2013; detailed information can be found in the Release Notes.
- RPPR Open Pilot to All Federal Demonstration Partnership (FDP) Institutions
The Research Performance Progress Report in eRA Commons will accommodate submission of non-SNAP awards, including multi-project (complex) and training awards, as part of a pilot open to members of the FDP. For more information on this, see NIH Guide Notice NOT-OD-13-113.
Training for non-SNAP RPPR training originally scheduled in October has been moved to Thursday, November 14, 2013 10:00 AM – 11:30 AM EST. For registration information on this training go to: https://www3.gotomeeting.com/register/262932326 - BioMedical Workforce (BMW) Initiative Tied to RPPR
Data from the new Personal Profile is used as part of the NIH initiative to track the diversity of the biomedical research workforce so that informed decisions can be made about training of the optimal number of people for the appropriate types of positions that will advance science and promote health. As part of the validation process for RPPR submissions, eRA Commons will provide warnings when information on the Personal Profile is missing from key personnel identified in the report. - Super Storm Sandy Reporting Link Now On the Status Screen
If you are one of the many institutions that received special administrative supplement funding as a result of the devastating effects of Super Storm Sandy, a link will now appear in eRA Commons Status screen to allow grantees to submit quarterly progress reports to a new reporting site and view these submissions. Grantees currently have the ability to submit these reports only by clicking on a link in an email notification. - eRA Commons No Longer Accepts Full Social Security Numbers
As part of the ongoing effort to protect your information, the Personal Profile or any other data fields in eRA Commons no longer accept a full Social Security Number (SSN) beyond four digits. - NIH Review Roster URL Has Changed
The public roster webpage "NIH Scientific Review Group (SRG) Roster Index" that hosts the rosters for NIH review meetings has moved to a new, more secure technology that has been developed by eRA and has a new URL. The old URL or the roster has changed as of October 24, to http://public.era.nih.gov/pubroster. Please update your bookmarks accordingly.
July 2013
The following are highlights of eRA's upcoming July 18-19 system-wide software release. These are just some of the many features being rolled out for this release; check out the release notes for more information on features and fixes.
- Launch of Redesigned Personal Profile section in eRA Commons
A sophisticated and intuitive personal profile section will make its debut in eRA Commons with this release. Some of the highlights: a dashboard to alert you to any missing information; ability to update your email address in multiple places at one time; short explanations for why certain data is being collected.
- Changes to Accommodate SBIR/STTR Reauthorization Act
Under this Act, all small business applicants are required to register with the Company Registry Database at www.sbir.gov at the time of application. Starting with this release, applicants will be given a warning if their registration file is not attached to an SBIR/STTR application.
A Reminder About RPPR
- Changes in Research Performance Progress Report (RPPR): With this release the option for initiating an eSNAP progress report will no longer be available. RPPR is a federally mandated reporting format for all federal grant agencies (NIH, NSF, DoD, etc.) designed to provide consistent information on the progress of federally funded research and research related activities. RPPR replaced the eSNAP progress reports for SNAP awards and PHS 416-6 for Fellowship progress reports in May, and will eventually replace the use of the PHS 2590 for non-SNAP awards. For more information, please see the RPPR web page and the Guide Notice NOT-OD-13-061.
April 2013
The following are highlights of eRA's upcoming April 18-19 system-wide software release.
- The Expansion of Research Performance Progress Report (RPPR) functionality
- Progress Report Additional Materials (PRAM) link on Status screen can appear in two formats:
- Public Compliance PRAM
- This link will appear on the Status screen upon submission of an RPPR with publications that are not in compliance with the public access policy (current PRAM functionality).
- IC Requested PRAM
- IC Requested PRAM link will appear when the funding Institute/Center sends a request for additional material, information or clarification concerning a submitted RPPR
- The grantee will submit a response to that request through the eRA Commons
- Public Compliance PRAM
- See Guide Notice NOT-OD-13-035 for more information about RPPR and PRAM.
- RPPR is replacing eSNAP for SNAP progress reports and the PHS 416-9 for Fellowship progress reports
- All SNAP and F progress reports with start dates of July 1st or later will be required to use RPPR
- For SNAP awards the initiate eSNAP option will remain available until July 2013; however, this is only to accommodate reports due before May 15th that are late.
- If you initiate an eSNAP for a report due on or after May 15th, contact the eRA Help Desk so that the eSNAP can be removed and an RPPR initiated in its place.
- Progress Report Additional Materials (PRAM) link on Status screen can appear in two formats:
- Other small issues within Commons have been fixed. See the Release Notes for detailed information on these fixes.
January 2013
The following are highlights of eRA's upcoming January 24-25 system-wide software release.
xTrain
- The Statement of Appointment (Form PHS 2271) has been modified to reflect the OMB approved revised list of Field of Training (FOT) codes and Specialty Boards
- xTrain will now recognize the amendment date when an appointment has been modified and the trainee is appointed or reappointed before the original end date. The system will no longer display an error message about overlapping appointment dates.
- The fellowship will now be displayed under Prior Support when the trainee has an appointment and previously had a fellowship.
- When fellowships are being terminated, only the years where the stipend amount is greater than zero and the status is either awarded or terminated will be displayed.
- New online help for xTrain. When you see a question mark (?) and click on it, it will take you to information about that page or content. The question marks appear on the following screens: My Grants, Trainee Roster, Trainee Appointments and Terminations, Statement of Training Appointment, Termination Notice NRSA, and List of Grants.
eRA Commons
- Expansion of Research Performance Progress Report (RPPR) with several fixes and enhancements added
ASSIST
- Application Submission System & Interface for Submission Tracking (ASSIST) pilot underway.
- Users are getting their first experience developing multi-project applications on-line in the new ASSIST system as they prepare to submit for January due date.
- Additional pilot opportunities will close over the next few months.
NIH will transition all multi-project applications to electronic submission by January 2014.
Scheduled Downtime for January Release:
eRA Commons will be unavailable beginning at 9 p.m. ET Thursday, January 24 and will return to service by 7 a.m. ET Friday, January 25.
After the January Release, make sure to review the Release Notes for detailed information on the changes.
2012
October 2012
The following are highlights of eRA’s upcoming October 18-19 system-wide software release.
- Use of Research Performance Progress Report (RPPR) Expanded
The use of RPPR is extended to all SNAP eligible progress reports and all Fellowships (F activity codes). For more information about RPPR, please visit the Research Performance Progress Report (RPPR) web page. - Progress Report Additional Materials (PRAM) Included in RPPR
The Progress Report Additional Materials (PRAM) feature provides a means for the grantee to enter, review, and submit information in response to specific request(s) from NIH for additional materials or in response to non-compliant publications following the submission of an RPPR. - Principal Investigator (PI) Status Screen Updates
A new status screen for Principal Investigators (PIs) will be included in the release. The new interface will be more consistent with the screen Signing Officials see. It will let the PI see all errors and warnings from submissions even after the application has moved forward in the review process. - xTrain Updates
There are several changes coming to xTrain in this release. The major improvements are:- Fixes the PDFs of xTrain appointments to ensure that important data and signatures are included.
- Enables the termination of appointments and fellowships when there is a change of institution. The name of the new institution, project title (if it has changed) and appropriate Business Official will be listed on the Termination Notice User Interface and PDF.
- In the near future, PDFs of xTrain appointments that are missing important data and signatures will be regenerated with all the correct information.
April 2012
New Features (April 2012)
- Electronic submission of Change of Institution (Type 7) applications – This process allows an institution to turn over an active grant to another institution. It is an open pilot, meaning that you have the choice to use the electronic process or the traditional paper process. There are two parts to the Change of Institution submission. The institution holding the grant must complete a Relinquishing Statement through eRA Commons that states it is giving up the grant and identifies the receiving institution. The receiving institution must submit an application via Grants.gov using the Parent Funding Opportunity Announcement listed at http://grants.nih.gov/grants/guide/parent_announcements.htm.
- Electronic submission of Successor-in-Interest (Type 6) Applications – Also in an open pilot, this process is similar to Change of Institution (Type 7) Applications. It applies in cases where an organization is taken over by another organization through a merger or corporate change. Please look for an NIH Guide Notice next week.
- Research Performance Progress Report (RPPR) – The RPPR was sparked by a federal mandate to create a progress report in a standard format across federal agencies so that recipient reporting is standardized. The RPPR begins as a limited pilot, with seven institutions volunteering to test the procedures. We anticipate a wider pilot over the summer and going to full production in 2013. Additional information can be found on the Research Performance Progress Report Web page and NIH Guide Notice NOT-OD-12-083.
New Fixes
- Various fixes for the Administrative Supplement (Type 3) system which is currently in open pilot.
- A fix for users experiencing difficulties in adding the role type of “Other” to the All Personnel section of the eSNAP.
January 2012
Submitting and Tracking Administrative Supplement Requests Electronically
As you may be aware, NIH is piloting two programs for the electronic submission of administrative supplement (non-competing Type 3) requests, one through a "streamlined system" in the eRA Commons and one through Grants.gov for the benefit of researchers at system-to-system institutions. The open pilot programs will begin on February 1, 2012 (See NIH Guide Notice NOT-OD-12-024).
eRA implemented changes to provide grantees the ability to create, edit, and submit an electronic version of the administrative supplement request using the eRA Commons. Whether submitted through the eRA Commons or through the Grants.gov portal, users will be able to track the administrative supplement requests in eRA Commons.




 eRA Intranet
eRA Intranet