How Does a PI See the Review Outcome?
Review Outcomes are found in Summary Statements. These statements are PDF documents combining reviewers' written comments and the Scientific Review Officer's (SRO*) summary of the discussion surrounding your application during the review meeting.
Policy: Scoring System and Procedure
As a PI, you can view your application's Summary Statement using the Status Information screen once it has been released by the SRO following the review meeting. If you are a signing official and are looking for the Summary Statement, see How Does an SO See the Review Outcome?
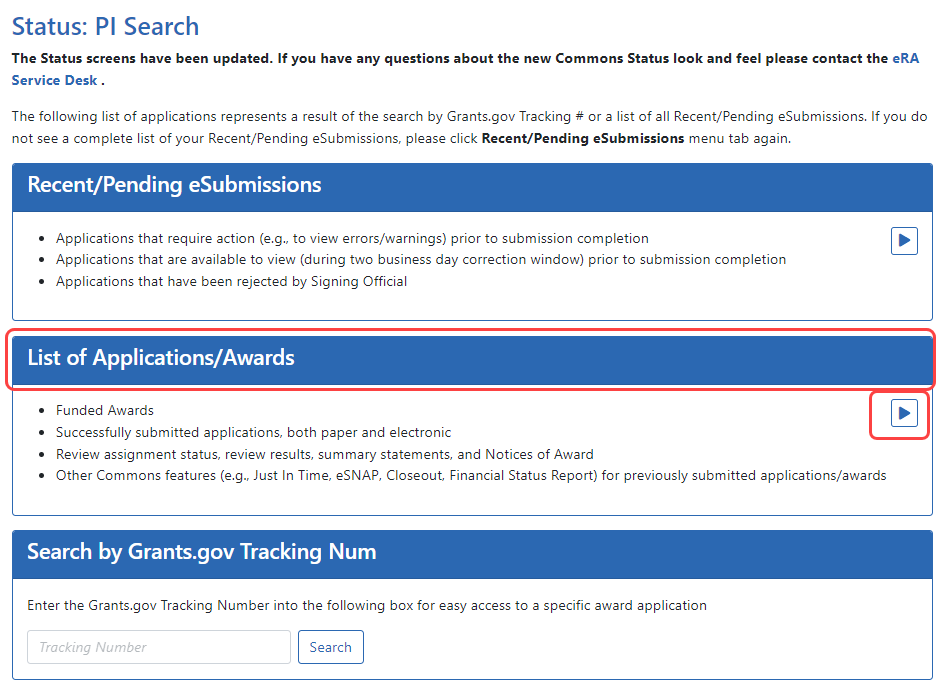
To view your Summary Statement:
- Log into Commons.
- Navigate to the Status module.
- Click the
 List of Applications/Grants section.
List of Applications/Grants section.The
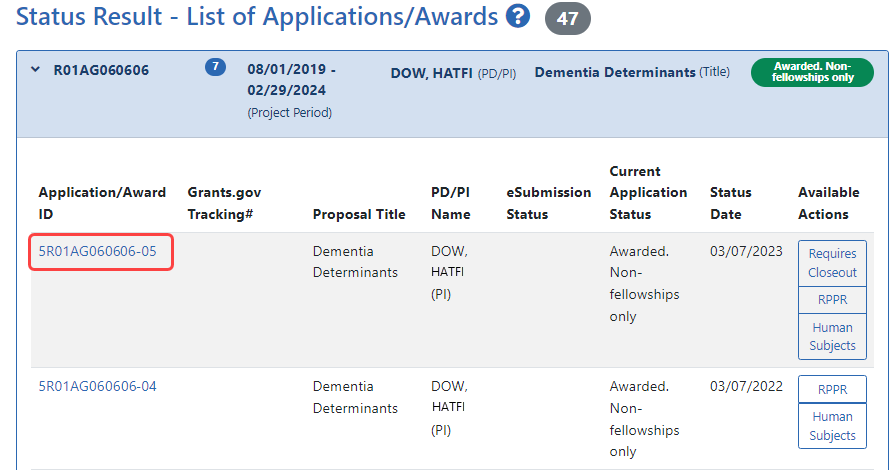
 results display in either a Grouped or Flat view format. You may toggle between those views as desired. The application ID will be provided as a link which, when selected, opens the Status Information screen.
results display in either a Grouped or Flat view format. You may toggle between those views as desired. The application ID will be provided as a link which, when selected, opens the Status Information screen. - Select the application ID link for the specific application. For a list of possible application statuses, see https://www.era.nih.gov/docs/era_status_codes.pdf.
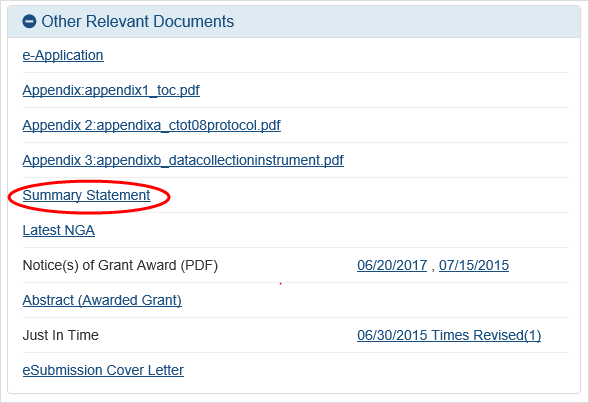
The Status Information screen displays. The Status Information screen includes a section called Other Relevant Documents. This section houses links to various application-related documents, including the Summary Statement.
-
Select the
 link titled Summary Statement. It will open in a separate window.
link titled Summary Statement. It will open in a separate window.
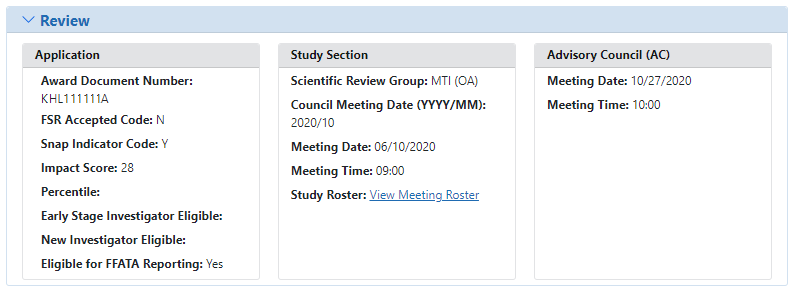
Abbreviated review information is found in the ![]() Review section of the Status Information screen. Expand this section to see the following:
Review section of the Status Information screen. Expand this section to see the following:
- Application:
- Award Document Number
- FSR Accepted Code
- Snap Indicator Code
- Impact Score
- Percentile
- Early Stage Investigator Eligible
- New Investigator Eligible
- Eligible for FFATA Reporting
- Study Section
- Scientific Review Group
- Council Meeting Date
- Meeting Date
- Meeting Time
- Study Roster
- Advisory Council
- Meeting Date
- Meeting Time
TIP: *Other Transaction Authority (OTA) - Some screens and terminology may be different in order to accommodate review of OTA, a type of award that is neither a grant nor a contract but a different way of funding that is being used across NIH. These changes will typically not be visible to NIH or agency reviewers.