Adding Studies
After the initial submission of the application, additional studies can be added once the summary statement is released.
Access HSS via the Human Subjects links in the Action column of Status or the Human Subjects link in section G.4.b in the progress report. To add studies, the HSS record must be in Work in Progress status. See How To Change the Application Status and Resubmit for instructions on changing the submission status.
Access HSS via the HSCT Post Submission tab via the Human Subjects links in the Action column of Status or the Human Subjects link in section G.4.b in the progress report. To add studies, the HSS record must be in Work in Progress status. See How To Change the Application Status and Resubmit for instructions on changing the submission status.![]() (click to view tab)
(click to view tab)
From the Application Information page, click on the HSCT Post Submission tab, then click the Edit button.
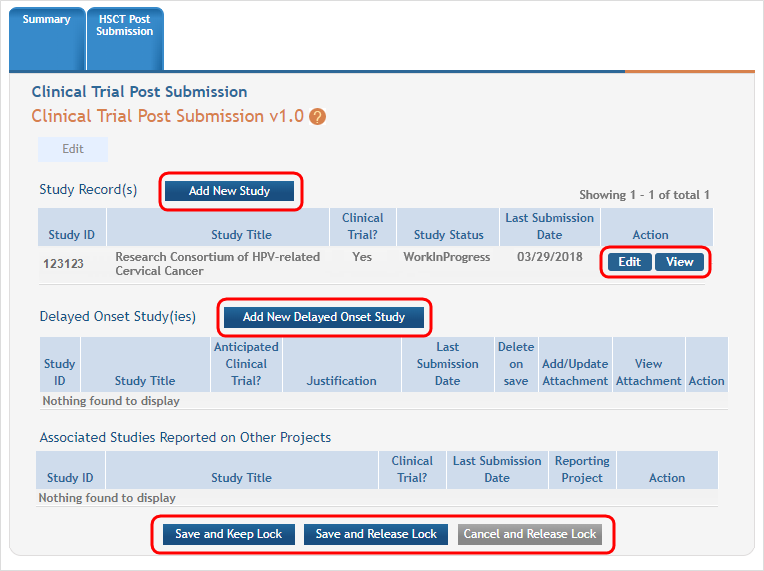
Any study records already submitted are displayed and can be viewed and buttons to Add New Study and Add New Delayed Onset Study are displayed. Click on the appropriate button to add studies. ![]() (click to view)
(click to view)
Once the study has been added be sure to use the Save and Keep Lock or Save and Release Lock buttons to secure your updates.