SBIR/STTR Foreign Disclosure Form—Request for Additional Materials
After your organization receives an award from SBIR/STTR, your organization will be required to submit a form detailing foreign affiliations or relationships to foreign countries at regular intervals and within 30 days of any substantive change. The Required Disclosures of Foreign Affiliations or Relationships to Foreign Countries form applies to all competing applications for funding under the NIH, CDC, and FDA SBIR and STTR programs submitted for due dates on or after September 5, 2023. For details and to find a copy of the form that needs to be completed, see Required Disclosures of Foreign Affiliations or Relationships to Foreign Countries.
Regular, annual updates are required at the time of all SBIR/STTR annual, interim, and final Research Performance Progress Reports (RPPRs). For changes that occur between RPPR submissions, updated disclosure forms are required within 30 days of any change in ownership, entity structure, covered individual, or other substantive changes in circumstance; see guide notice Clarification of Implementation of the NIH SBIR and STTR Foreign Disclosure Pre-award and Post-Award Requirements.
While either a principal investigator (PI) or a signing official (SO) can upload a form, write comments, and save the request, only the SO can actually submit the form to the awarding agency. The PDF form must be flattened; see Flattening PDFs for Submission in Commons.
To upload a Foreign Disclosure Form:
- Log in to eRA Commons as a signing official (SO) or principal investigator (PI).
Either an SO or PI can upload the form, but ONLY the SO can submit it. - Navigate to the Status module and search for an award that requires the foreign disclosure form.
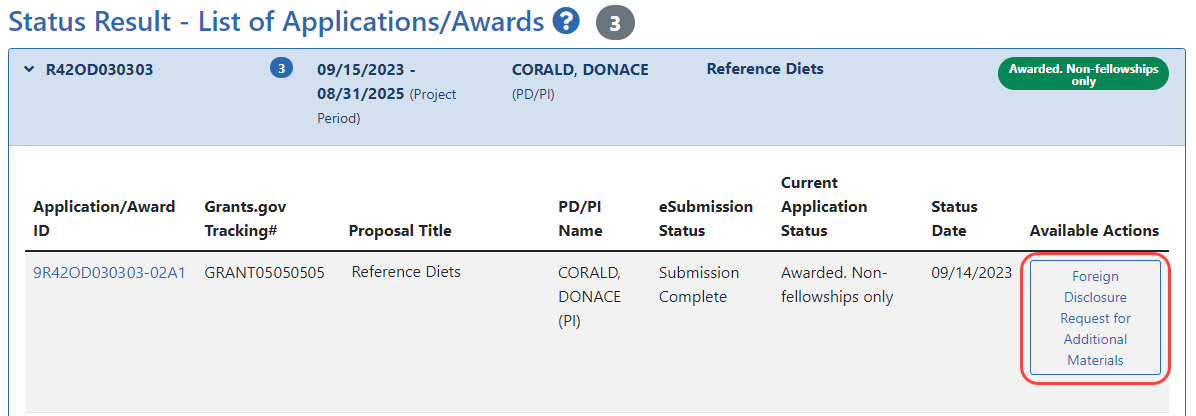
- On the award, click the Foreign Disclosure Request for Additional Materials link, which appears for all SBIR/STTR awards.
For a PI, this link appears in the Available Actions column in the list of Applications/Awards. For an SO, this link appears
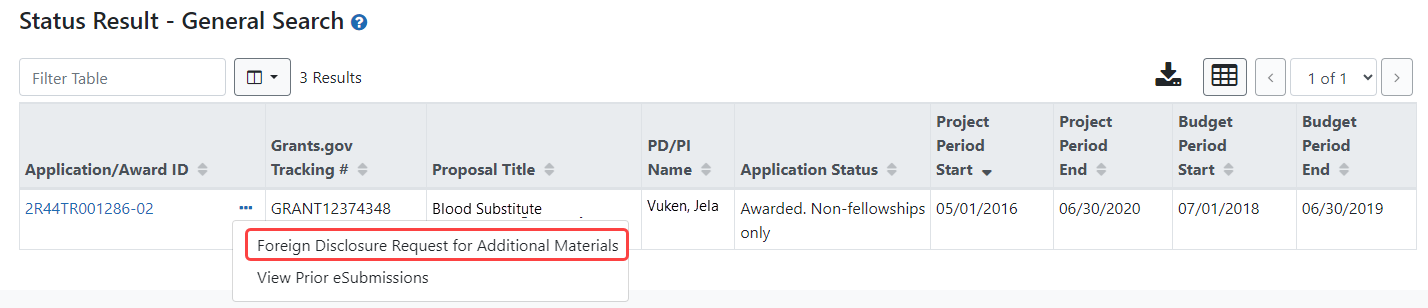
the Available Actions column in the list of Applications/Awards. For an SO, this link appears  under the three-dot ellipsis icon for an award in the table of search results.
under the three-dot ellipsis icon for an award in the table of search results.
The Foreign Disclosure Request for Additional Materials screen appears. In the explanatory text, you can see a 'foreign disclosure form' link (outlined below), which leads to a page with details and a copy of the blank form.
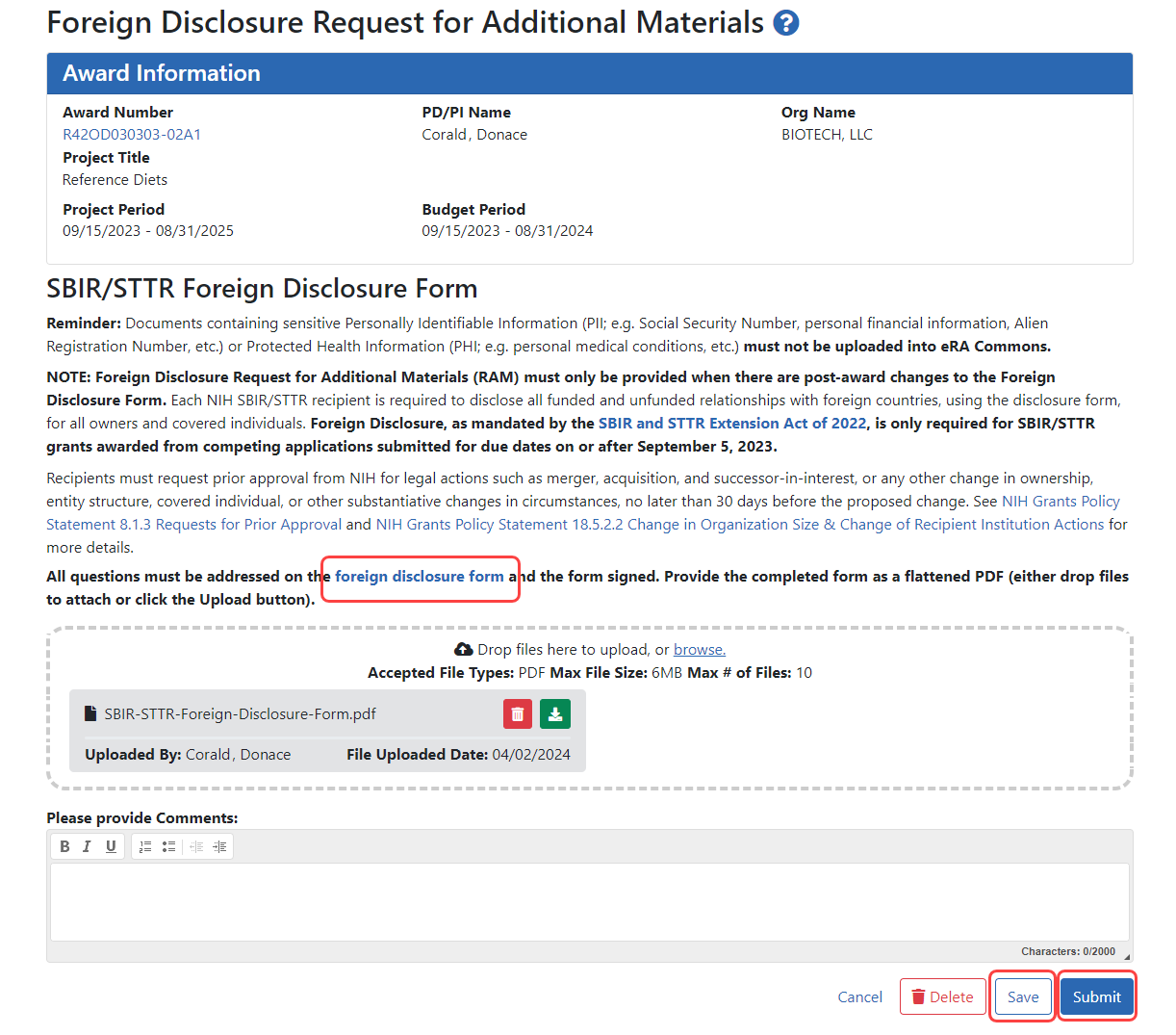
- Drag and drop file(s) in the "Drop Files Here.." area, or click the browse link and choose file(s) from your drive. The drop files area specifies how many files and what file types are allowed. Uploaded files are saved immediately with the request. For uploaded files, you can:
- Click the Download icon
 and the browser will open or download the file.
and the browser will open or download the file. - Click the Delete icon
 to remove the file.
to remove the file.
- Click the Download icon
-
Click Save to save the comments in the request.
-
When the form is ready to submit, an SO should log in and click the Submit button. (The PI does not see or have access to the Submit button.)
A green success message lets you know that the file has been submitted. After submission, you cannot make changes. The form immediately becomes available for agency staff to review and process after submission.