Interim Report Additional Materials (IRAM)
The awarding agency might issue the awardee a request for Interim Report Additional Materials (IRAM) following the submission of an Interim RPPR (research performance progress report). This type of request lets the awardee enter, review, and submit information in response to a specific request for additional information from the program official at the awarding agency.
Either the principal investigator (PI) or signing official (SO) (or their delegates) can upload files and provide a revised project outcome in response to an IRAM request. For a multi-PI project, the contact PI can respond to the IRAM. However, only a SO can submit the IRAM package to the agency.
Agency-Specific Instructions: Department of Commerce (DOC). Interim Report Additional Materials are not applicable for DOC awards.
About Revised Outcomes
For Revised Outcome, please review the instructions at the top of the IRAM screen as well as the following information.
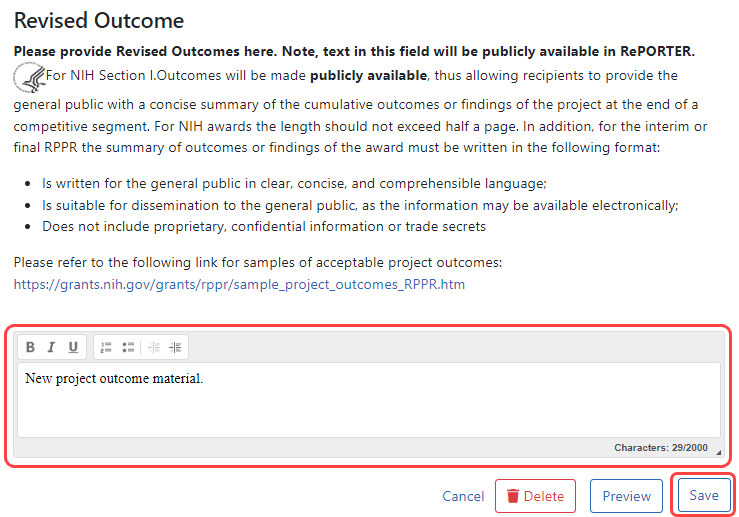
To submit revised project outcomes, ![]() enter text in the Revised Outcome text box on the Interim Progress Report Additional Materials (IRAM) screen. The Save button at bottom of screen saves the text you enter.
enter text in the Revised Outcome text box on the Interim Progress Report Additional Materials (IRAM) screen. The Save button at bottom of screen saves the text you enter.
Project outcomes provide information regarding the cumulative outcomes or findings of the project. Note that outcomes are made available to the general public once approved by the program official, allowing recipients to provide a concise summary with of the cumulative outcomes or findings of the project at the end of a competitive segment. The name of the PI will be attached to the public posting in RePORTER.
For NIH awards the length of the outcome statement should not exceed 2000 characters. In addition, the summary of outcomes or findings of the award must be written according to these guidelines:
-
Is written for the general public in clear, concise, and comprehensible language
-
Is suitable for dissemination to the general public, as the information may be available electronically
-
Does not include proprietary, confidential information or trade secrets
Please refer to the following link for samples of acceptable project outcomes: https://grants.nih.gov/grants/rppr/sample_project_outcomes_RPPR.htm
See NIH notices NOT-OD-17-022 and NOT-OD-17-037 for additional details on this requirement.
Accessing the IRAM Screen
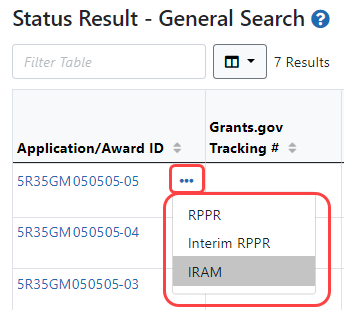
You can access an IRAM by clicking an IRAM action in the Status module. The SO view and the PI view of the Status module are different.
SOs access the Status module, search for awards, ![]() click the three-dot ellipsis menu on an award, and select IRAM.
click the three-dot ellipsis menu on an award, and select IRAM.
PIs access the Status module, expand an awards section, and ![]() click the IRAM button in the Available Actions column.
click the IRAM button in the Available Actions column.
Using the IRAM Screen
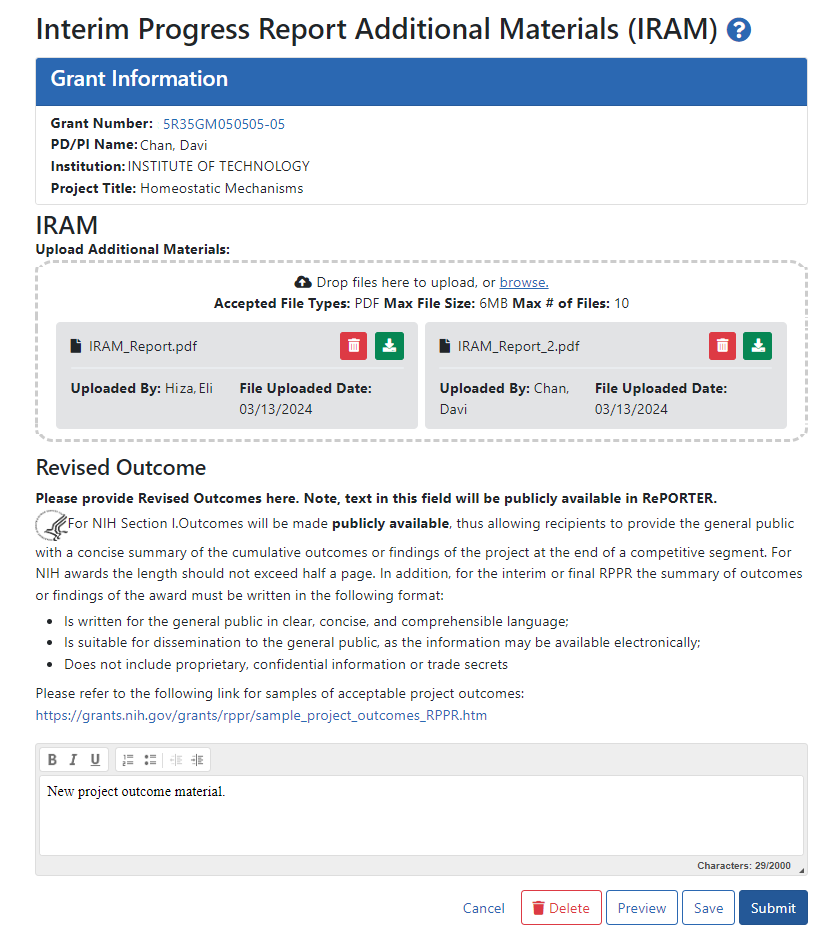
The IRAM screen appears after you click an award's IRAM action. Prepare your response to the IRAM and save it in up to 10 PDF files that are each smaller than 6MB. If you have questions about what to include in your response, consult with your program official.
On the IRAM screen, you can:
-
Drag or browse to files to upload using the "Drop files here to upload or browse" section. The files you upload are instantly saved to eRA Commons system, but are not available to the agency until they are submitted.
-
Remove files by clicking the trash can icon
 .
. -
Download/view files by clicking the download icon
 .
. -
Enter up to 2000 characters as a revised outcome, using the guidelines that are provided onscreen and in this topic.
-
Click the Save button to save the text entered into the Revised Outcome text box.
-
Click the Preview button to preview the entire submission package. Clicking Preview downloads a file that has a summary page listing files uploaded and the uploader's name and time uploaded, followed by the new Revised Outcome and the contents of the uploaded files.
-
Click Delete to delete the submission package
-
Click Submit to submit the IRAM package to the awarding agency, where the PO can review it. Only SOs have a Submit button; PIs cannot submit IRAM to the agency.