Information to guide you through the submission of the Final RPPR, or Interim RPPR, as part of the Closeout process. Also, information on how the Final RPPR is different from the Annual RPPR.
The Final Research Performance Progress Report (Final RPPR) is one of three reports required as part of the closeout process for the end of an award. A Final RPPR is required for any grant that is terminated and any award that will not be extended through award of a new competitive segment.
If a competitive renewal (Type 2) application has been submitted, the recipient must submit an Interim RPPR while their renewal application is under consideration. In the event that the Type 2 is funded, NIH will treat the Interim RPPR as the annual performance report for the final year of the previous competitive segment. If the Type 2 is not funded, the Interim RPPR will be treated by NIH staff as the institution's Final RPPR
Basic Tasks (Step-by-step instructions from the online help)*
- Determine which grants require closeout
- View grant closeout requirements under Closeout Status
- Submitting Your Interim Research Performance Progress Report
- Submitting Your Final Research Performance Progress Report
* You must be logged into eRA Commons with appropriate role(s) to complete these activities.
Main Screenshots
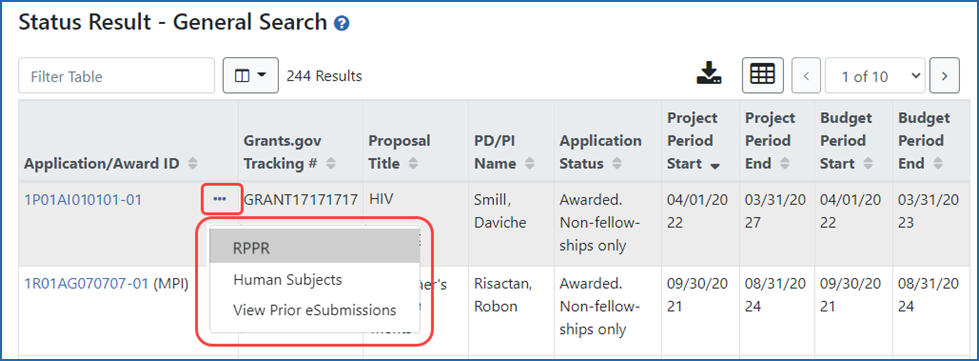
Figure 1: Status Result screen showing how to access the RPPR to edit information
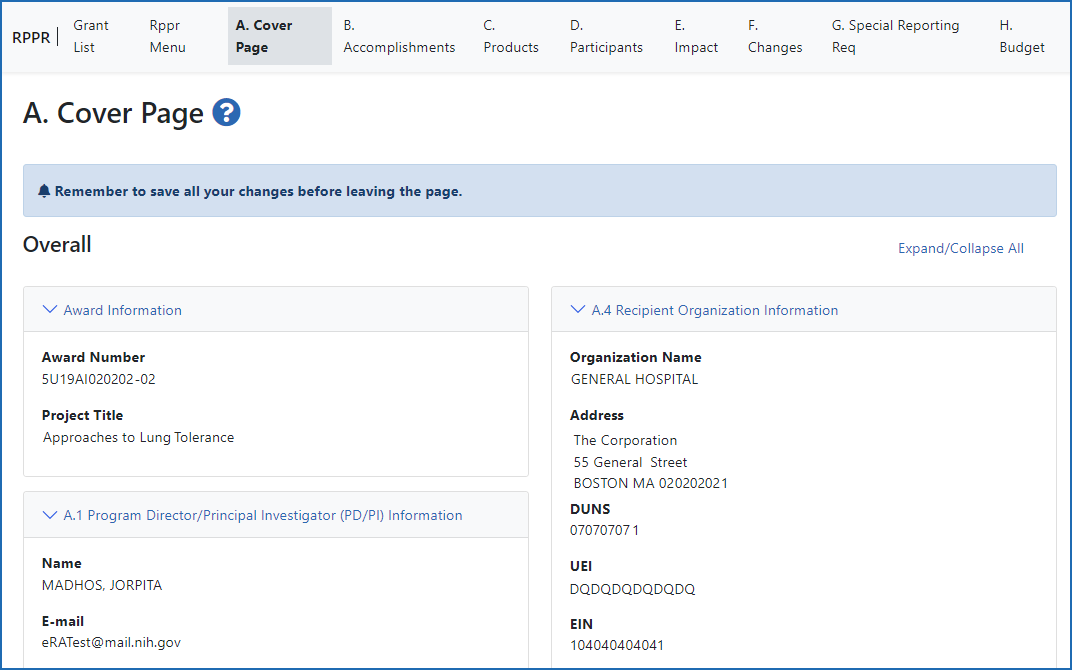
Figure 2: The RPPR form, section A, Cover Page and navigation tabs
Additional Resources
- RPPR Instruction Guide
- RPPR Resources Page
- Grants Closeout FAQs
- Research Performance Progress Report web page
- Overview of the Interim RPPR
- RPPR: Who Can Do What? (PDF - 76KB) (September 2018)
- eRA Commons Roles & Privileges At a Glance (PDF - 25 KB) (August 2018)
Policy
- NIH Grants Policy Statement: Closeout
- NIH Grants Policy Statement: Final Progress Report
- NIH Guide Notice NOT-OD-17-037: NIH Implementation of the Interim-RPPR While a Renewal Application is Under Consideration




 eRA Intranet
eRA Intranet